Introduction
2024 Cancer PAIN
Introduction
2024 CANCER PAIN
This second section of the 2024 PolyAnalgesic Consensus Conference (PACC)® project is focused on providing evidence-based guidance of best practices, and clinical pharmacology principles and evidence, related to intrathecal drug delivery for management of cancer pain.
It emphasizes that while evidence-based strategies are preferred, expert opinions are necessary when research data is limited. The cancer pain patient population is highly diverse, ranging from those with advanced disease and limited survival time, to individuals with stable conditions and longer life expectancy. The guideline aims to address unique factors related to cancer patients, technical and pharmacological considerations for IDD, and system-level factors that can ensure the successful and safe implementation of an IDD program.
Authors were chosen using the same criteria outlined in the 2024 non-cancer PACC section above. This group recognizes that the cancer pain population is a heterogenous group; with a spectrum from advanced disease, and limited survival, to stable disease and potential extended survival. The published literature and established evidence and consensus-based expert opinion were used to develop best practice recommendations, with the intention of updating these with future iterations based on any new evidence.

Reprinted from Neuromodulation: Technology at the Neural Interface, Timothy R Deer, Salim M Hayek, Jay S Grider, Jason E Pope, Shane E Brogan, Amitabh Gulati, Jonathan M Hagedorn, Natalie Strand, Jennifer Hah, Tony L Yaksh, Peter S Staats, Christophe Perruchoud, Nebojsa Nick Knezevic, Mark S Wallace, Julie G Pilitsis, Tim J Lamer, Eric Buchser, Vishal Varshney, Jill Osborn, Vasudha Goel, Brian A Simpson, Jose A Lopez, Denis Dupoiron, Michael F Saulino, Gladstone C McDowell 2nd , Fabian Piedimonte, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain, Pages No., Copyright (2024), with permission with permission from the International Neuromodulation Society.
Figure 1. The PACC consensus points review factors that impact the safe and effective delivery of IDD in cancer patients. IDD = intrathecal drug delivery, MRI = magnetic resonance imaging, PACC = Polyanalgesic Consensus Conference.
Materials and Methods
Development Process
The International Neuromodulation Society (INS) established the Polyanalgesic Consensus Conference (PACC) to improve patient care and access to neuromodulation techniques, focusing on Intrathecal Drug Delivery (IDD) for pain management. The PACC was composed of INS members selected for their clinical expertise, research contributions, and familiarity with the literature on IDD. The group regularly evaluated the latest evidence in peer-reviewed literature to develop consensus-based recommendations aimed at enhancing IDD’s efficacy and safety.
The development involved forming working groups responsible for conducting literature reviews, evaluating the evidence, and assigning evidence rankings. The recommendations were formulated and reviewed by section leaders and other non-conflicted PACC members. A consensus was reached through various communication methods, with a minimum of 80% agreement required. The final consensus points were graded according to the strength of evidence and applicability to clinical practice.
Management of Conflict of Interest (COI)
The INS implemented a strict COI management policy to ensure the impartiality of guideline development. Authors were required to disclose any conflicts, and one of the co-primary authors, without relevant conflicts, served as the adjudication officer. Authors with conflicts recused themselves from discussions or decisions related to affected recommendations. All recommendations were audited for bias by conflict-free members.
Literature Search, Evidence Ranking, and Consensus Development
The working groups conducted a comprehensive search of literature in English across various databases (e.g., Medline, EMBASE, Cochrane CENTRAL, BioMed Central, Web of Science, Google Scholar, PubMed, Current Contents Connect, Meeting Abstracts, and Scopus) to compile evidence for IDD in pain management. The identified literature was evaluated using modified United States Preventive Services Task Force (USPSTF) criteria1. Each study was assigned a letter grade based on quality, and a “level of certainty regarding benefit” was determined.
For each major topic, the PACC developed consensus points representing clinical guidance based on best practices and peer-reviewed evidence from sources such as randomized controlled trials (RCTs), observational studies, and case series. These consensus points should not be mistaken for recommendations based solely on expert opinion but are informed by a combination of evidence and clinical interpretation.
Table 1. Grade Using the United States Preventative Services Task Force Criteria Modified for Neuromodulation
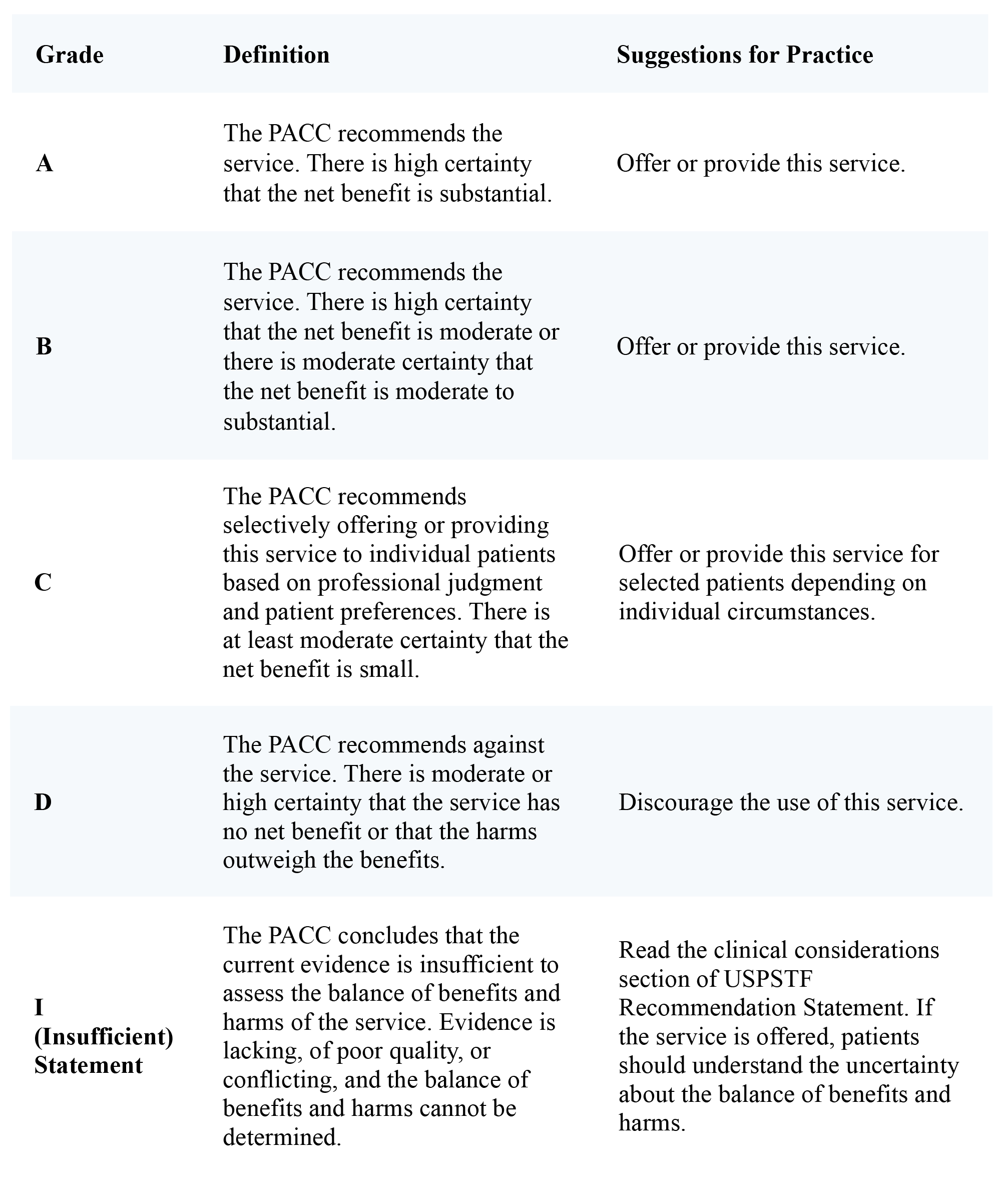
Reprinted from Neuromodulation: Technology at the Neural Interface, Timothy R Deer, Salim M Hayek, Jay S Grider, Jason E Pope, Shane E Brogan, Amitabh Gulati, Jonathan M Hagedorn, Natalie Strand, Jennifer Hah, Tony L Yaksh, Peter S Staats, Christophe Perruchoud, Nebojsa Nick Knezevic, Mark S Wallace, Julie G Pilitsis, Tim J Lamer, Eric Buchser, Vishal Varshney, Jill Osborn, Vasudha Goel, Brian A Simpson, Jose A Lopez, Denis Dupoiron, Michael F Saulino, Gladstone C McDowell 2nd , Fabian Piedimonte, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain, Pages No., Copyright (2024), with permission with permission from the International Neuromodulation Society.
Table 2. Levels of Certainty Regarding Net Benefit and Quality of Evidence with Evidence Levelsi
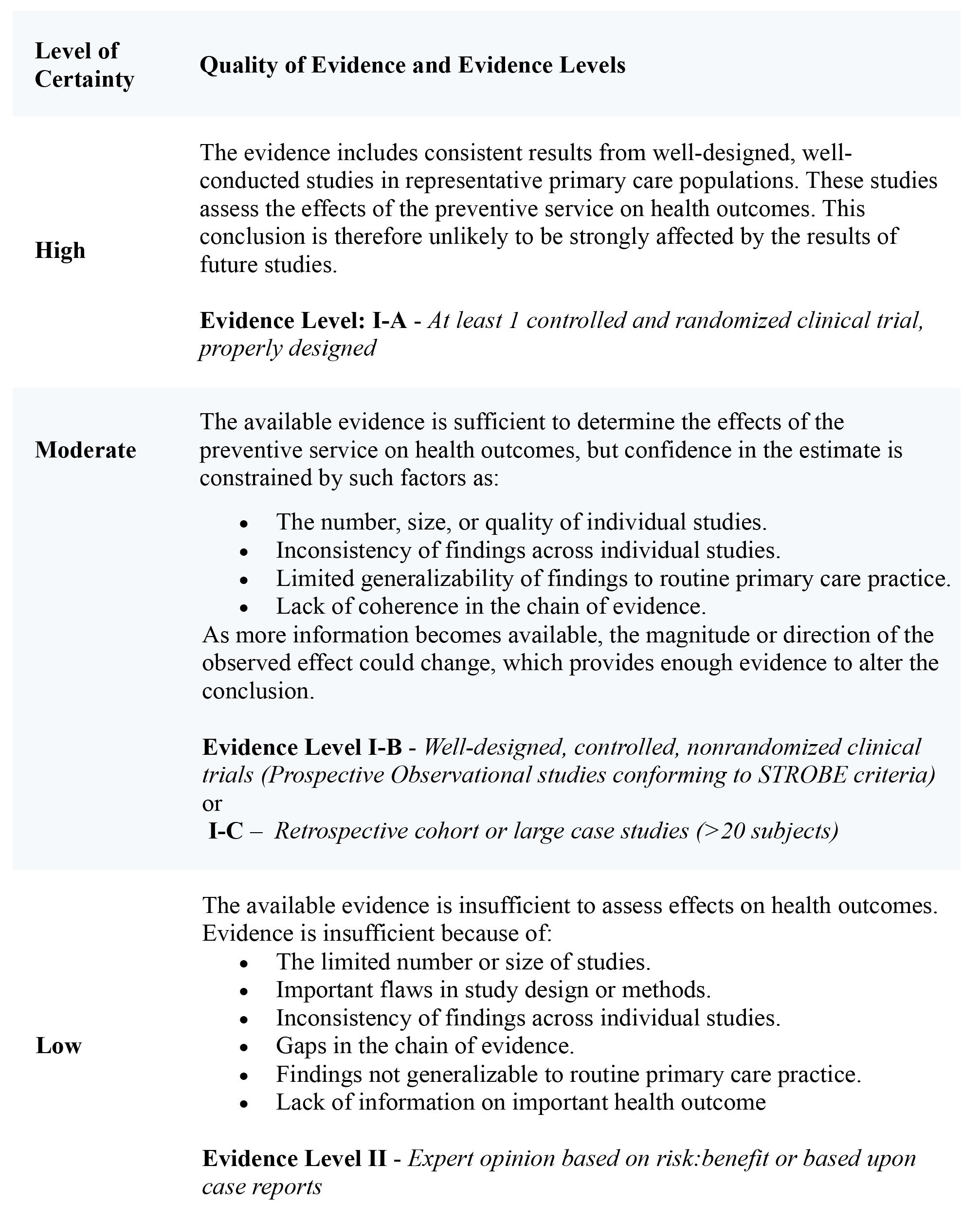
Reprinted from Neuromodulation: Technology at the Neural Interface, Timothy R Deer, Salim M Hayek, Jay S Grider, Jason E Pope, Shane E Brogan, Amitabh Gulati, Jonathan M Hagedorn, Natalie Strand, Jennifer Hah, Tony L Yaksh, Peter S Staats, Christophe Perruchoud, Nebojsa Nick Knezevic, Mark S Wallace, Julie G Pilitsis, Tim J Lamer, Eric Buchser, Vishal Varshney, Jill Osborn, Vasudha Goel, Brian A Simpson, Jose A Lopez, Denis Dupoiron, Michael F Saulino, Gladstone C McDowell 2nd , Fabian Piedimonte, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain, Pages No., Copyright (2024), with permission with permission from the International Neuromodulation Society.

More effective management of intrathecal drug delivery.
© Copyright 2025. All rights reserved.

More effective management of intrathecal drug delivery devices.
© Copyright 2025. All rights reserved.
