Introduction
2024 Non Cancer PAIN
Introduction
2024 NON CANCER PAIN
The PolyAnalgesic Consensus Conference (PACC)®
In 1981 Onofrio and Yaksh described the implantation of an intrathecal pump for the treatment of chronic pain in a patient with cancer and subsequently the use of intrathecal drug delivery (IDD) for cancer and non-cancer expanded over the next several decades.2,3 In 1999 the International Neuromodulation Society (INS) convened the inaugural PolyAnalgesic Consensus Conference in New York City with invited distinguished clinical experts in IDD to produce guidelines for intrathecal therapy.4,5 The first several meetings and publications of the guidelines ranked intrathecal medication combinations on the basis of the best available evidence and consensus.
In 2012 the guidelines categorized pain as either neuropathic or nociceptive and assigned medications based on the type of pain. However, they did not account for patient-specific factors. They also addressed issues like trialing, catheter-tip granulomas, and strategies to reduce morbidity and mortality associated with IDD. The 2017 updated guidelines incorporated patient-specific factors such as prognosis, age, starting opioid doses, and pain distribution (diffuse vs. localized). They also revisited trialing and safety/risk mitigation strategies.
The INS (International Neuromodulation Society) continued to convene the PACC to improve patient care and access to neuromodulation therapies. Experts in clinical practice, literature, and research were selected globally to assess the evidence supporting the efficacy and safety of neuromodulation techniques. The development process followed these steps:
- Literature Review & Evidence Evaluation: Working groups conducted literature searches on key topics. Authors reviewed and ranked the evidence, then formulated recommendations based on the findings. These recommendations were reviewed by multiple PACC members to ensure thorough assessment and avoid conflicts of interest.
- Consensus Development: Consensus was reached when ≥80% of contributing authors agreed on a recommendation. If a recommendation received <50% consensus, it was considered not supported.
- Recommendations & Best Practices: The document provides consensus points on IDD (intrathecal drug delivery) and optimizing outcomes. These are intended as best practices, not standards of care, and require clinical judgment for application.
Management of Conflict of Interest
To ensure transparency, the INS adhered to the ICMJE (International Committee of Medical Journal Editors) disclosure policies. All authors were required to disclose potential conflicts, with some authors recusing themselves from recommendations influenced by financial interests. The INS ensured a majority of conflict-free authors to maintain unbiased guidance.
LITERATURE
Search & Evidence Ranking
The evidence for neuromodulation therapies was gathered from various databases (e.g., Medline, PubMed, Cochrane etc.) using key terms related to intrathecal drug delivery and pain treatments. The evidence was rated using the USPSTF (U.S. Preventive Services Task Force) criteria and modified for neuromodulation (Table 1). Each topic had a consensus point supported by evidence, with guidance provided when evidence was lacking.
The 2024 guidelines build on previous updates, considering the latest safety data and publications aimed at improving efficacy. They emphasize the evolving practices in managing chronic pain with IDD.
The INS differentiates between the management of chronic noncancer pain and cancer pain. The published document focuses on non-cancer pain management, with a separate manuscript published for cancer-related pain. This app provides summarized information and the figures and graphs from the published article.
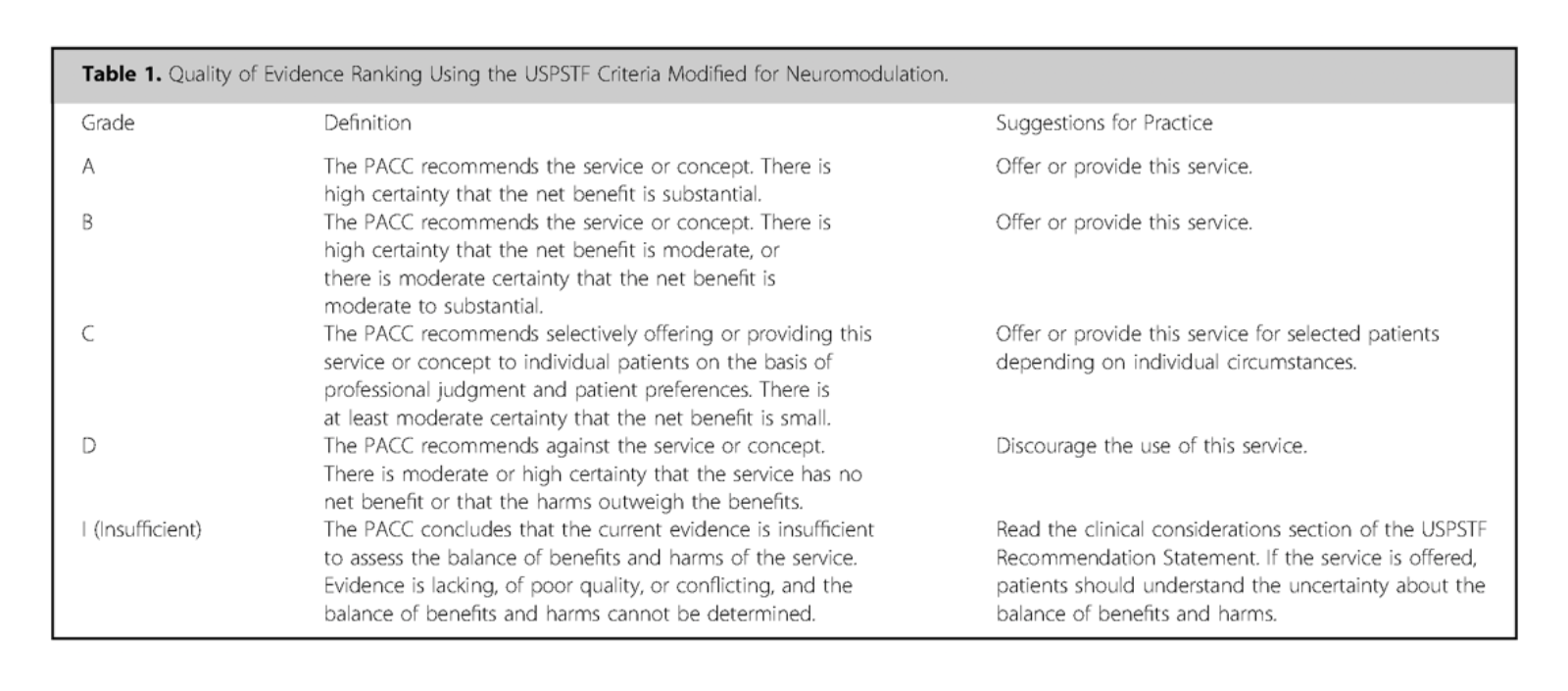
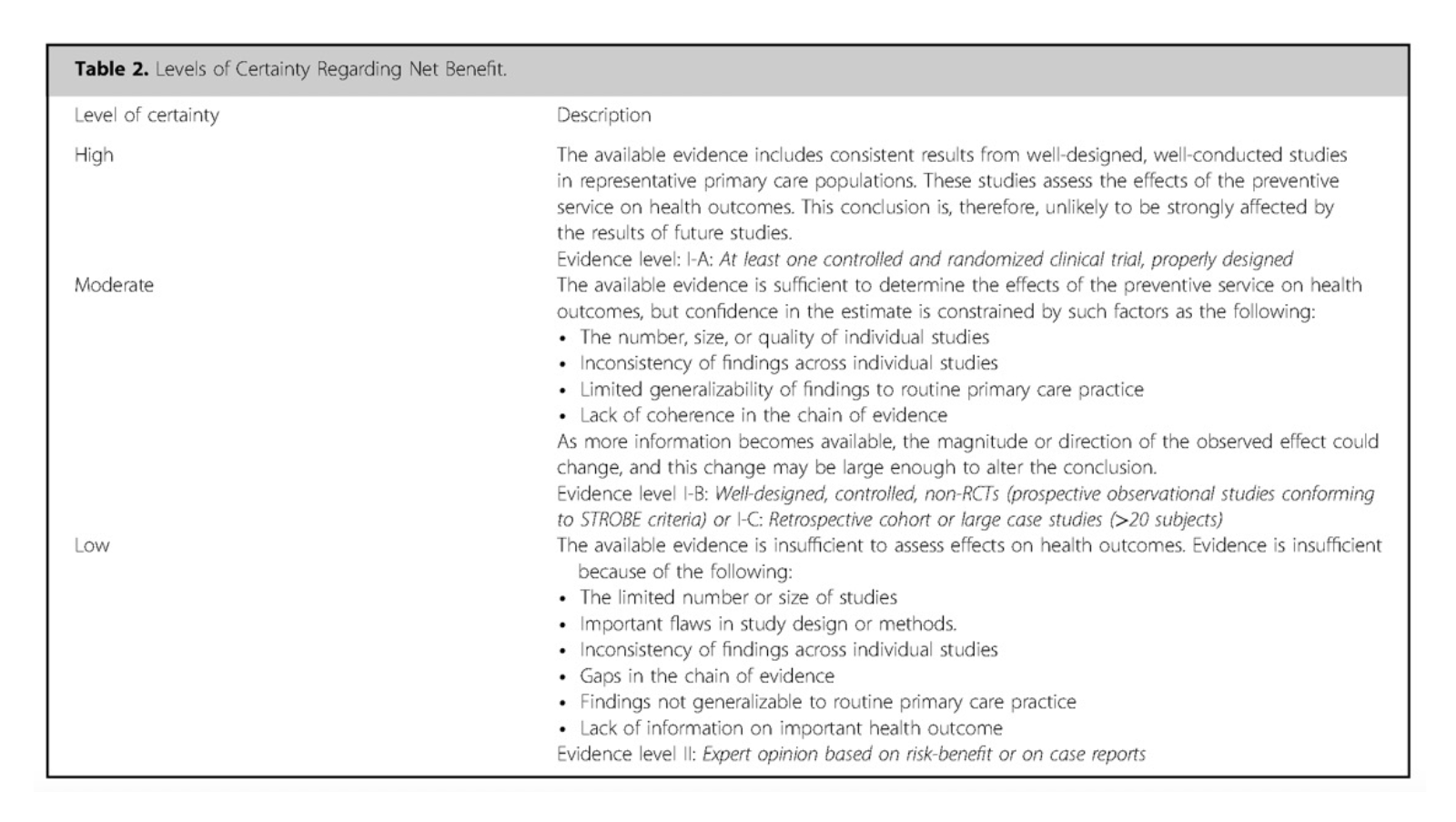

More effective management of intrathecal drug delivery.
© Copyright 2025. All rights reserved.

More effective management of intrathecal drug delivery devices.
© Copyright 2025. All rights reserved.
