Common IDD Management Issues
2024 Non Cancer PAIN
Common IDD
Management Issuess
2024 NON CANCER PAIN
Opioid Dose Escalation
Opioid dose escalation is a frequent issue in IDD for both cancer and non-cancer pain, indicating tolerance or diminishing analgesic response. Several factors, such as patient age, starting opioid dose, and co-administration of bupivacaine, influence dose escalation. Younger patients generally exhibit a steeper escalation curve66,134,135. Evidence suggests that patients on low opioid doses at baseline are less prone to dose escalation post-implantation11. The baseline opioid dose plays a crucial role, and lower doses are recommended when possible, especially for chronic non-cancer pain. Sex has not been found to significantly affect dose escalation135,136.
Consensus Point 17: Clinicians considering IDD should comprehend patient factors favoring a good analgesic response, including older age and low opioid dosing at baseline. USPSTF grade B; level of certainty moderate; evidence level IB
Bupivacaine is frequently used as an adjuvant to opioids to control dose escalation.
Studies on the addition of bupivacaine have produced mixed results, with some showing no extra pain relief, while others indicate a blunting of opioid escalation136,137.
The combination of bupivacaine with fentanyl appears to offer a safer and equally effective alternative compared to hydromorphone, which is associated with granuloma formation70,10.
Consensus Point 18: A combination of opioid with bupivacaine, regardless of when the local anesthetic is added, appears to blunt opioid dose escalation and might be beneficial from the outset of IDD. In case of low opioid dosing and localized pain in patients with chronic noncancer pain, fentanyl may be a good option in combination with bupivacaine. USPSTF grade B; level of certainty moderate; evidence level IB
Loss of Efficacy
Over time, IDD patients may experience a perceived loss of analgesic efficacy, which can result from various factors such as disease progression, psychologic adaptation, medication tolerance, hyperalgesia or system-related issues (e.g., catheter disruption, granuloma formation).
A sudden loss of efficacy should prompt evaluation of the device149, including assessing for MRI exposure, recent trauma, or pump refill issues. In cases of gradual decline in pain relief, potential degradation of medication delivery, tolerance, worsening pathology, or psychologic factors should be considered.
Management Strategy: Conduct a thorough workup for IDDS malfunction before escalating intrathecal dosing. This approach should include evaluation of pump settings, drug delivery integrity, and assessment for granuloma or outflow obstruction causes (Fig 1).
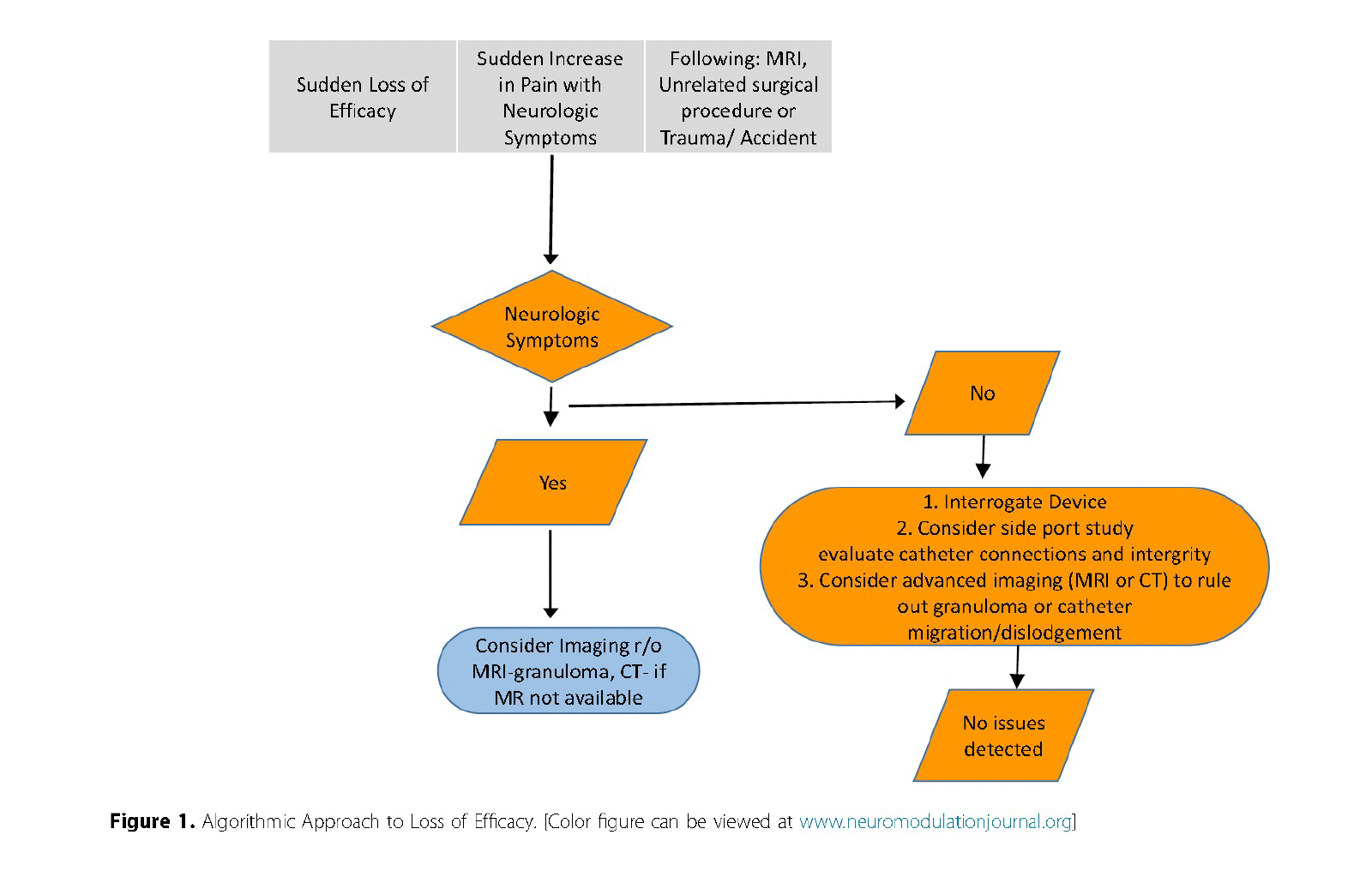
Reprinted from Neuromodulation: Technology at the Neural Interface, Vol 27 / Issue 7, Timothy R Deer, Salim M Hayek, Jay S Grider, Jonathan M Hagedorn, Gladstone C McDowell 2nd Philip Kim, Denis Dupoiron, Vasudha Goel, Rui Duarte, Julie G Pilitsis, Michael S Leong, Jose De Andrés, Christophe Perruchoud, Harry Sukumaran, Alaa Abd-Elsayed, Michael Saulino, Dennis Patin, Lawrence R Poree, Natalie Strand, Karina Gritsenko, Jill A Osborn, Ivano Dones, Anjum Bux, Jay M Shah, Brad L Lindsey, Erik Shaw, Tony L Yaksh, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain, Pages 1107-1139., Copyright (2024), with permission from the International Neuromodulation Society.
Rotation to Other Opioids
Opioid medications, though effective for pain management in various contexts, present significant challenges when used long-term due to tolerance, dependence, and potential for addiction. Over time, patients often require higher doses to achieve the same analgesic effects, a phenomenon that can lead to escalating dosages and increased risk of side effects. In light of these concerns, strategies such as opioid rotation have been explored to maintain effective pain relief at lower dosages, thereby minimizing adverse outcomes152.
Opioid rotation involves switching from one opioid to another while discontinuing the initial opioid. The rationale behind this approach lies in the concept of incomplete cross-tolerance among opioid medications. Each opioid has a unique receptor interaction and pharmacodynamic profile, leading to varying degrees of tolerance and side effects among individual agents150,151. By rotating to a different opioid, it is possible to achieve comparable or improved analgesia with a lower total dose, thereby mitigating tolerance and side effects.
A systematic review assessing opioid rotation in patients with cancer pain, who were treated with oral, transdermal, or intravenous opioids, reported that the intervention improved pain control or reduced side effects in 50% to 70% of patients . For patients with intrathecal drug delivery systems (IDDS), a retrospective study involving 37 cases demonstrated that rotating from morphine to hydromorphone improved pain relief by at least 25% in approximately 50% of patients experiencing inadequate pain control with morphine. Additionally, hydromorphone use was associated with a reduction in side effects such as nausea, vomiting, pruritus, and sedation152 .
Although these findings are promising, there is a lack of randomized controlled trials (RCTs) specifically evaluating the therapeutic benefits of opioid rotation for intrathecal drug administration.
Equianalgesic tables, which provide estimated dose conversions based on opioid potency, are commonly used to guide clinicians in determining the initial dose of the new opioid. However, these tables lack robust scientific evidence, particularly for long-term opioid therapy, and may be even less reliable when applied to the intrathecal space—especially in the case of lipophilic opioids 155-159.
To navigate this uncertainty, clinicians should adopt a cautious approach, initiating the new opioid at a lower dose than that predicted by equianalgesic tables, then titrating upward based on patient response. This conservative strategy helps minimize the risk of overdose and adverse effects, given that most patients require a lower dose of the new opioid than calculated 160-162.
Monitoring and patient education are essential components of this process to ensure safety and optimize pain management outcomes.
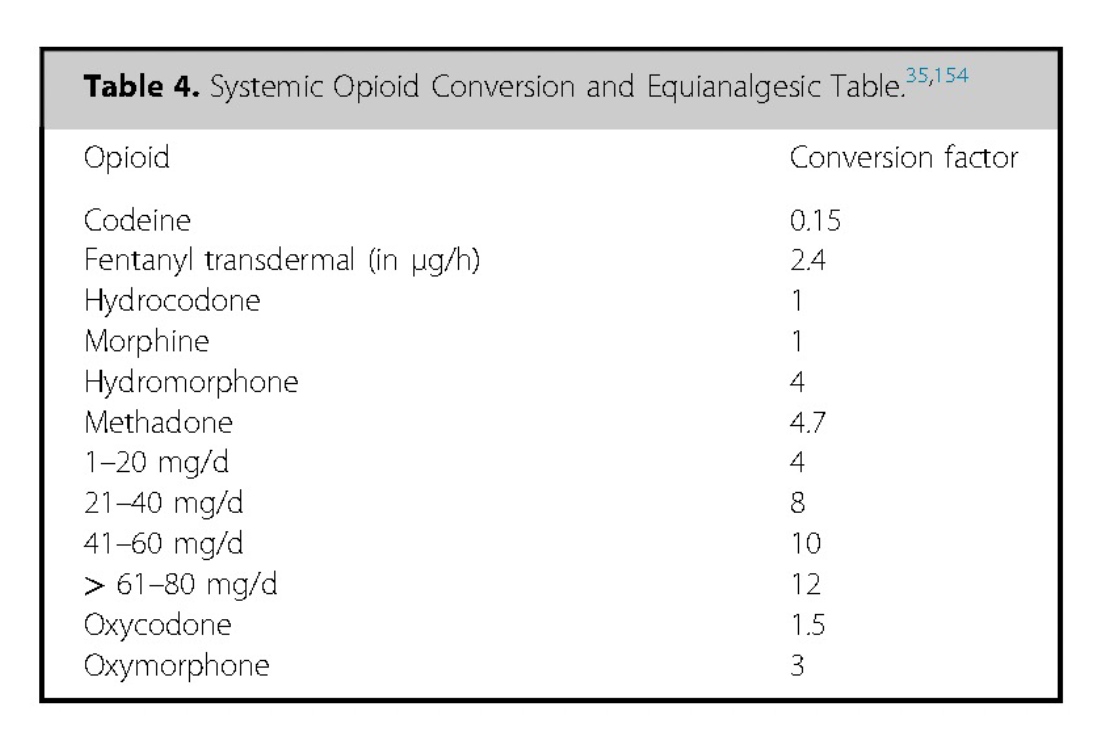
Reprinted from Neuromodulation: Technology at the Neural Interface, Vol 27 / Issue 7, Timothy R Deer, Salim M Hayek, Jay S Grider, Jonathan M Hagedorn, Gladstone C McDowell 2nd Philip Kim, Denis Dupoiron, Vasudha Goel, Rui Duarte, Julie G Pilitsis, Michael S Leong, Jose De Andrés, Christophe Perruchoud, Harry Sukumaran, Alaa Abd-Elsayed, Michael Saulino, Dennis Patin, Lawrence R Poree, Natalie Strand, Karina Gritsenko, Jill A Osborn, Ivano Dones, Anjum Bux, Jay M Shah, Brad L Lindsey, Erik Shaw, Tony L Yaksh, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain, Pages 1107-1139., Copyright (2024), with permission from the International Neuromodulation Society.
Consensus Point 19: When performing an opioid rotation with IDDS, the equianalgesic dosage should be investigated and the starting dosage of the new opioid should be decreased considerably, to consider the potential difference in receptor response, and then titrated to achieve appropriate analgesia. It should be noted that in many countries, morphine is the only opioid labeled for use, and this rotation may require off-label decision-making. USPSTF grade B; level of certainty moderate; evidence level II
Low-Dose Intrathecal Opioid Therapy
The concept of low-dose intrathecal drug delivery (IDD) emerged in the mid-2000s, initially described in anecdotal reports of efficacy by pain management specialists128. Prior to this, IDD was typically initiated when systemic opioid treatment reached an endpoint characterized by reduced efficacy, clinician discomfort with dose escalation, or unacceptable side effects. The approach of low-dose IDD hinges on the following principles:
- Combining systemic and intrathecal opioids can compound the risks associated with each modality.
- IDD is more effective after achieving an opioid-free steady-state.
- Effective pain management can often be achieved at significantly lower doses of intrathecal opioids compared to those used previously.
An early retrospective case series128 and subsequent studies, including a prospective observational study by Hamza et al.124, demonstrated that low-dose IDD (approximately 1.4 mg/day of intrathecal morphine) could provide effective pain relief, with most patients discontinuing systemic opioids shortly after implantation. Further research by Grider et al. suggested that low-dose opioid monotherapy is more effective for mechanical low back pain than for neuropathic or mixed pain syndromes129. It is also suggested that older patients may respond more favorably to microdosing, though further validation is needed.
Although no randomized controlled trials (RCTs) have yet been conducted, low-dose IDD therapy has gained traction as a potential option, and recommendations now suggest starting opioid doses within the low-dose range10,11. Additionally, evidence suggests that high systemic opioid use can impair neuromodulation efficacy and is associated with greater patient dissatisfaction and a higher likelihood of device explantation163-165.
Consensus Point 20: In combination with systemic opioid reduction or elimination, low-dose mono-opioid therapy may be useful in treating mechanical axial back pain. USPSTF grade B; level of certainty moderate; level of evidence IB.
Consensus Point 21: Addition of bupivacaine at the outset of therapy may be beneficial in blunting opioid dose escalation or in treating those patients with mixed neuropathic and mechanical pain. USPSTF grade C, level of certainty moderate, level of evidence IB
Ziconotide Dosing and Side-Effect Management
Proper individualized dosing is critical for ziconotide efficacy. The PolyAnalgesic Consensus Conference (PACC) recommends a starting dose of 0.5 to 1.0 µg/day (0.02–0.04 µg/hour)11which may require a compounding pharmacist to perform drug dilution. Some clinicians may opt for a more aggressive starting dose of 2.4 µg/day (0.1 µg/hour)122. A maximum dose of 19.2 µg/day (0.8 µg/hour) is indicated by the FDA-approved labeling166.
For long-term management, dose increases of no more than 0.5 µg/day are recommended, with weekly reassessment for response. Time to analgesic effect is longer compared to smaller molecules like morphine, given ziconotide’s larger molecular weight and slower movement through tissues169.
Consensus Point 22: The starting dose for intrathecal ziconotide infusion should be 0.5 to 1.0 μg/d (0.02–0.04 μg/h). USPSTF grade A; level of certainty high; evidence level I.
Consensus Point 23: When titrating intrathecal ziconotide dosing, an increase of ≤0.5 μg/d with weekly reassessment is warranted. USPSTF grade B; level of certainty moderate; evidence level I.
When titrating, alternative dosing strategies like patient-controlled analgesia have been described, but no prospective randomized studies have been performed121. Nocturnal dosing122, and intermittent bolusing have been explored. While no single dosing strategy has proven superior, these methods may offer additional flexibility in managing patient needs.
Side-Effect Management
Common side effects of ziconotide include nausea, dizziness, confusion, memory loss, hallucinations, nightmares, and a 1% incidence of psychotic thoughts171,172. These side effects are often dose-dependent, and reducing the dose usually resolves them, especially if the reaction is minor. The PACC recommends reduction to a previously tolerated effective dose without side effect10. With more severe reactions the drug may be safely discontinued as there is no risk of withdrawal175.
Consensus Point 24: When side effects occur, it is recommended to reduce the intrathecal infusion dose of ziconotide to the last tolerated level that relieved pain without side effects. In the setting of severe side effects, it also is recommended that ziconotide can be discontinued without the risk of withdrawal. USPSTF grade B; level of certainty moderate; evidence level II.
Rare occurrences of muscle breakdown, new muscle pain and weakness and elevated serum creatine kinase (CK) have been reported176.The PACC recommends obtaining a baseline CK level and repeating if symptoms develop.
Consensus Point 25: A baseline CK level should be obtained before intrathecal ziconotide infusion is initiated and whenever symptoms of muscle weakness or pain manifest with long-term infusion. The presence of new muscle pain and/or weakness should be considered an indication to obtain this laboratory study. USPSTF grade B; level of certainty moderate; evidence level II.
In the event of a ziconotide overdose, immediate cessation of therapy and supportive care is warranted, as no reversal agents are currently available.
REFILL PROCESS
Reservoir refill is a crucial component of intrathecal drug delivery therapy, performed either in a clinical setting or at home by a qualified nurse. The refill interval, or the time between consecutive reservoir refills, is influenced by the concentration of the drug and the daily dose, and typically ranges from several weeks to a few months. The refill interval will also be shortened if patients use patient-directed intrathecal boluses, as this increases drug consumption. Regular use of such boluses can cause the low reservoir alarm to activate sooner, while less frequent use can extend the interval177,178.
To avoid complications, refills should be scheduled with sufficient residual volume in the reservoir before the low-reservoir alarm is triggered. Failure to do so can result in “low reservoir syndrome” and withdrawal symptoms179. It is critical for patients to be aware of their next scheduled refill appointment and the anticipated alarm date180. High concentrations of certain intrathecal medications may increase the risk of granuloma formation near the catheter tip141,181.
Refill Procedure – The refill procedure involves palpating the pump externally, using a template to guide the needle into the reservoir chamber, and aspirating the remaining solution from the previous refill. The aspirated volume should match the calculated volume indicated by the pump programmer. The new drug solution is then instilled through the same needle.
The absence of an internal reservoir volume sensor means that the volume programmed by the clinician must precisely match the actual volume instilled. Incorrect programming the volume can result in false low reservoir alarms or withdrawal symptoms. During titration, dose increases may require more frequent refills. In these cases, a higher concentration drug solution can extend the interval between refills. When changing drug concentrations, it is crucial to program a “bridge bolus” to account for the residual solution in the pump and catheter, ensuring accurate dosing and preventing under- or over-dosing.
Risks and Complications – Inadvertent injection of an intrathecal solution into subcutaneous tissue—referred to as “pocket fill”—is a rare but potentially serious complication that can expose the patient to a large drug load182. This error can be mitigated by using fluoroscopy or ultrasound to assist with accurate port localization and needle placement, although ultrasound use in IDD delivery is considered off-label183,184,185,186,187. If the actual aspirated volume is less than expected, potential causes include misprogramming, pocket fill, or unexpected overinfusion. Overinfusion warrants immediate pump replacement to prevent overdose or withdrawal. Conversely, a greater-than-anticipated aspirated volume might suggest catheter obstruction, pump failure, or misprogramming, all of which require further diagnostic evaluation.
Evaluation of IDD System Failure
Initial Imaging: The evaluation typically begins with plain radiography to visualize the entire catheter, connectors, and entry into the spinal canal. If the catheter is radiolucent or if findings are inconclusive, a catheter access port (CAP) aspiration may be necessary183. Aspiration of cerebrospinal fluid (CSF) must be successful before contrast medium can be injected and visualized using fluoroscopy or computed tomography (CT)189. This helps diagnose catheter fractures, tip loculations, or migration into subdural or epidural spaces149. A priming bolus is necessary after catheter CSF aspiration and study to avoid withdrawal syndrome and Hagedorn et al have described an algorithm for use with contrast agents during these studies193.
Computed Tomography (CT) and MRI: CT imaging can provide structural information, especially when combined with contrast agents, to better visualize the relationship of the catheter to other anatomical structures. For potential granulomas, MRI with gadolinium contrast is the preferred diagnostic modality, with CT myelography as an alternative if MRI is unavailable.
Radionuclide Scintigraphy: Indium 111 diethylenetriamine pentaacetate (DTPA) can be used as a tracer to evaluate catheter patency. This technique requires sequential scanning over two to three days and is less accessible and more costly, with limited anatomical resolution and a high false-negative rate. It has not been fully FDA-approved for intrathecal pump use.
Magnetic Resonance Imaging (MRI): MRI can detect soft tissue abnormalities, abscesses, and granulomas near the catheter tip, which may cause neurological injury due to spinal cord compression. MRI is considered the gold standard for detecting granulomas at the catheter tip or CT myelogram if MRI cannot be performed 8,12.
Consensus Point 26: In troubleshooting for decreased efficacy of IDD, a stepwise approach should be used while recognizing the need for urgent interventions in appropriate situations. USPSTF grade B; level of certainty moderate; evidence level II

More effective management of intrathecal drug delivery.
© Copyright 2025. All rights reserved.

More effective management of intrathecal drug delivery devices.
© Copyright 2025. All rights reserved.
