Trialing in Cancer Pain
2024 Cancer PAIN
Trialing in Cancer Pain
2024 CANCER PAIN
Trialing in cancer pain is controversial due to the lack of prospective evidence supporting its necessity16,20. Data shows that only 21.6% of cancer pain patients undergo trialing before implant161. Given the established efficacy of IDD in cancer pain and cost-effectiveness, trialing should be discretionary, based on clinician preference, patient condition, and insurance requirements. Table 6 presents additional information on trialing for cancer patients.
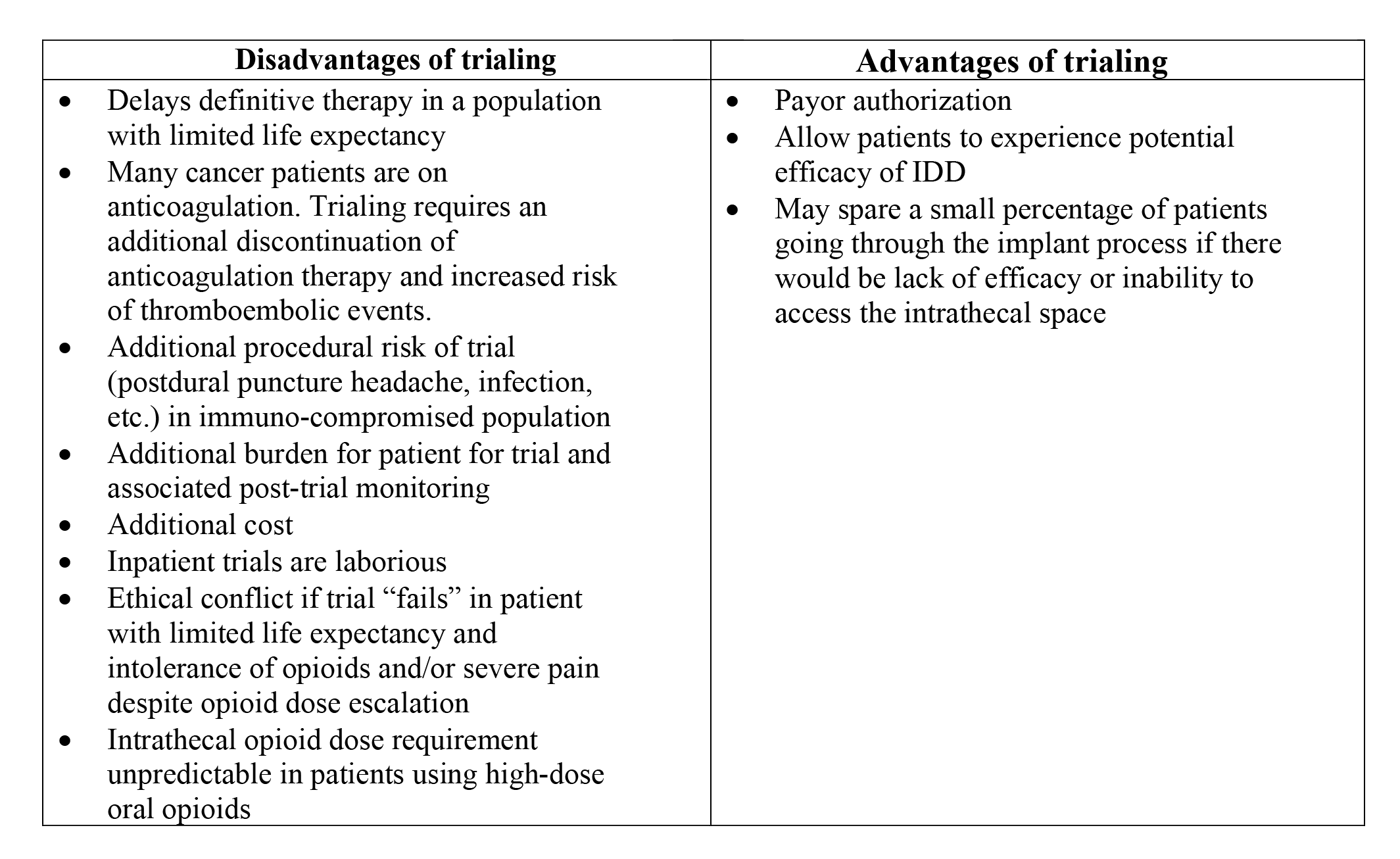
Reprinted from Neuromodulation: Technology at the Neural Interface, Timothy R Deer, Salim M Hayek, Jay S Grider, Jason E Pope, Shane E Brogan, Amitabh Gulati, Jonathan M Hagedorn, Natalie Strand, Jennifer Hah, Tony L Yaksh, Peter S Staats, Christophe Perruchoud, Nebojsa Nick Knezevic, Mark S Wallace, Julie G Pilitsis, Tim J Lamer, Eric Buchser, Vishal Varshney, Jill Osborn, Vasudha Goel, Brian A Simpson, Jose A Lopez, Denis Dupoiron, Michael F Saulino, Gladstone C McDowell 2nd , Fabian Piedimonte, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain, Pages No., Copyright (2024), with permission with permission from the International Neuromodulation Society.
Consensus Point 14. Given the individuality of each patient and clinical presentation, the PACC recommends that trialing before intrathecal pump implantation for cancer-related pain should be optional and at the discretion of the physician and patient. USPSTF Grade B; Level of certainty low; Quality of evidence 1-C.

More effective management of intrathecal drug delivery.
© Copyright 2025. All rights reserved.

More effective management of intrathecal drug delivery devices.
© Copyright 2025. All rights reserved.
