Perioperative Management
for IDDS Procedures
2024 Non Cancer PAIN
Perioperative Management
for IDDS Procedures
2024 NON CANCER PAIN
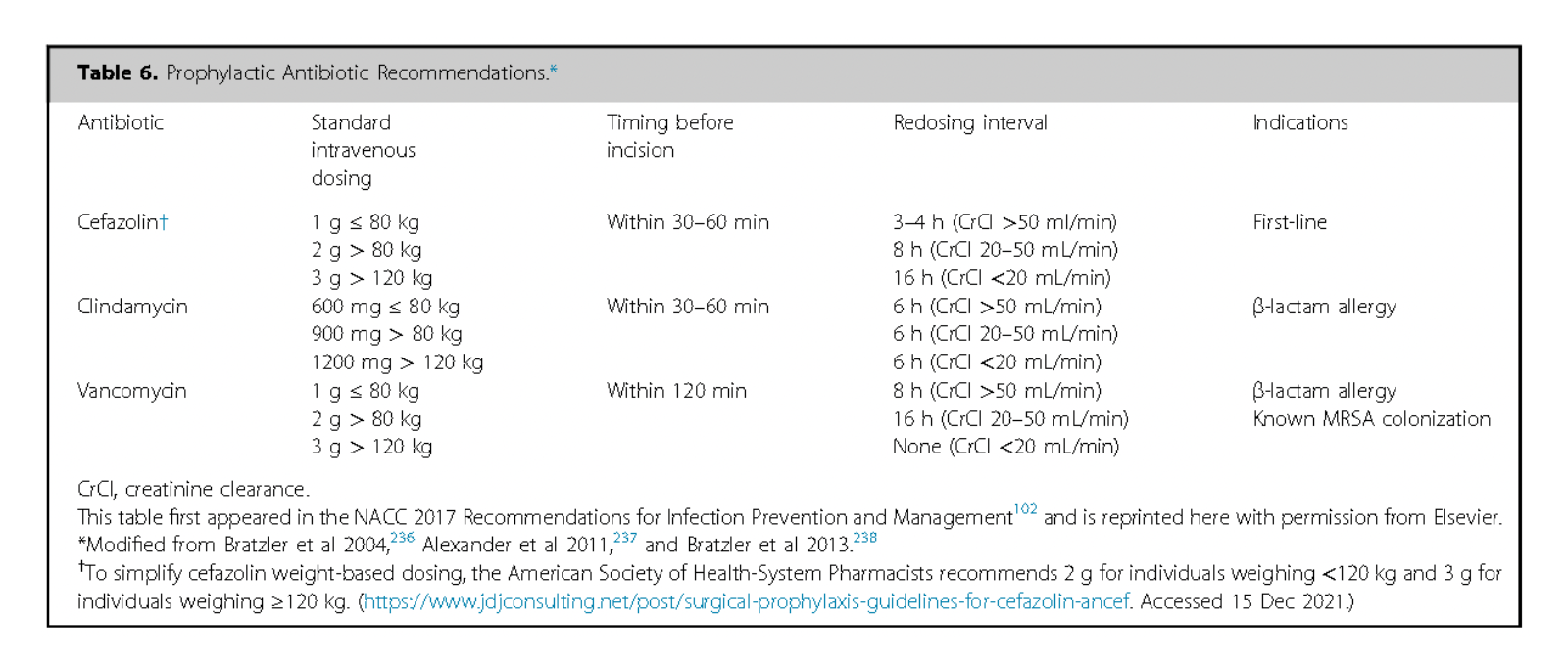
Successful use of implanted drug administration systems depends on careful and thorough perioperative medical and surgical evaluation, along with meticulous decision-making, to ensure both immediate and long-term effectiveness.
Reprinted from Neuromodulation: Technology at the Neural Interface, Vol 27 / Issue 7, Timothy R Deer, Salim M Hayek, Jay S Grider, Jonathan M Hagedorn, Gladstone C McDowell 2nd Philip Kim, Denis Dupoiron, Vasudha Goel, Rui Duarte, Julie G Pilitsis, Michael S Leong, Jose De Andrés, Christophe Perruchoud, Harry Sukumaran, Alaa Abd-Elsayed, Michael Saulino, Dennis Patin, Lawrence R Poree, Natalie Strand, Karina Gritsenko, Jill A Osborn, Ivano Dones, Anjum Bux, Jay M Shah, Brad L Lindsey, Erik Shaw, Tony L Yaksh, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain, Pages 1107-1139., Copyright (2024), with permission from the International Neuromodulation Society.
Preoperative Considerations
Obtain comprehensive medical history to evaluate the patient’s ability to tolerate anesthesia and surgery. Focus on cardiopulmonary health with assessment of heart and lung function. Ensure adequate renal and hepatic function. Check for immune system status and potential infection risk.
Conduct a thorough physical examination, especially of the skin at the implantation site, checking for abrasions or infection.
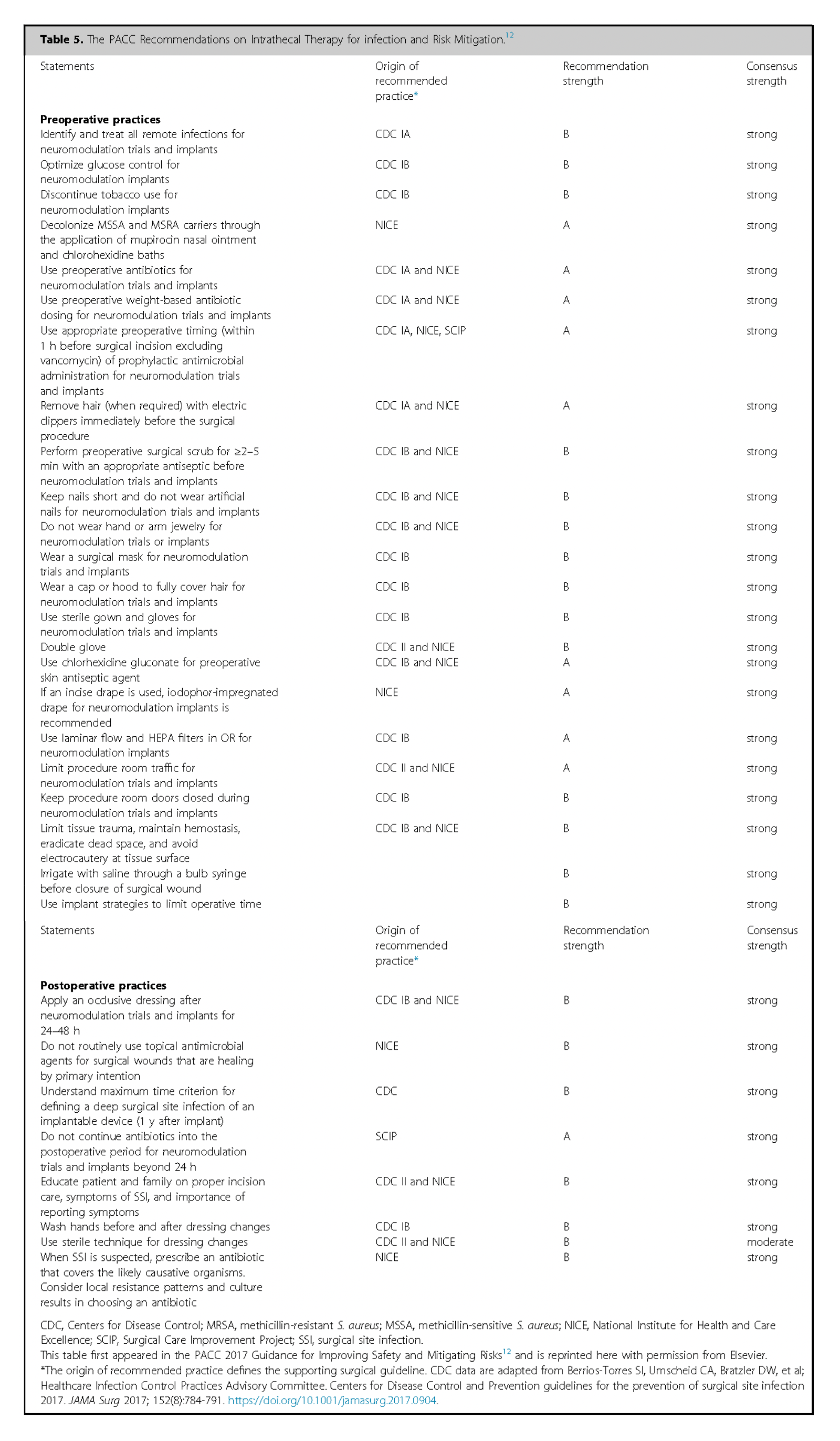
Preoperative lab tests should include:
- Complete Blood Count (CBC)
- Basic Metabolic Panel
- Coagulation Profile
- C-Reactive Protein
- Creatine Kinase (CK)(if using ziconotide)
- Urinalysis
Establish a policy for routine nasal swabbing for Methicillin-Sensitive Staphylococcus Aureus (MSSA) and Methicillin-Resistant Staphylococcus Aureus (MRSA).
Screen for obstructive sleep apnea using tools like Epiworth Sleepiness Scale226, STOP-BANG score227. Ensure compliance with Continuous Positive Airway Pressure (CPAP) or Bilevel Positive Airway Pressure (BiPAP) therapy.
Hemoglobin A1C (HbA1c) should ideally be <8% 228, 229. Monitor blood sugar closely before surgery, as elevated glucose may increase infection risk.
Advise smoking cessation for at least two months before surgery 230, 231.
Evaluate the immune status and consider nutritional support if the patient is malnourished or immunocompromised. For patients on long-term steroids, evaluate risks of delayed wound healing and potential to taper medications preoperatively 232.
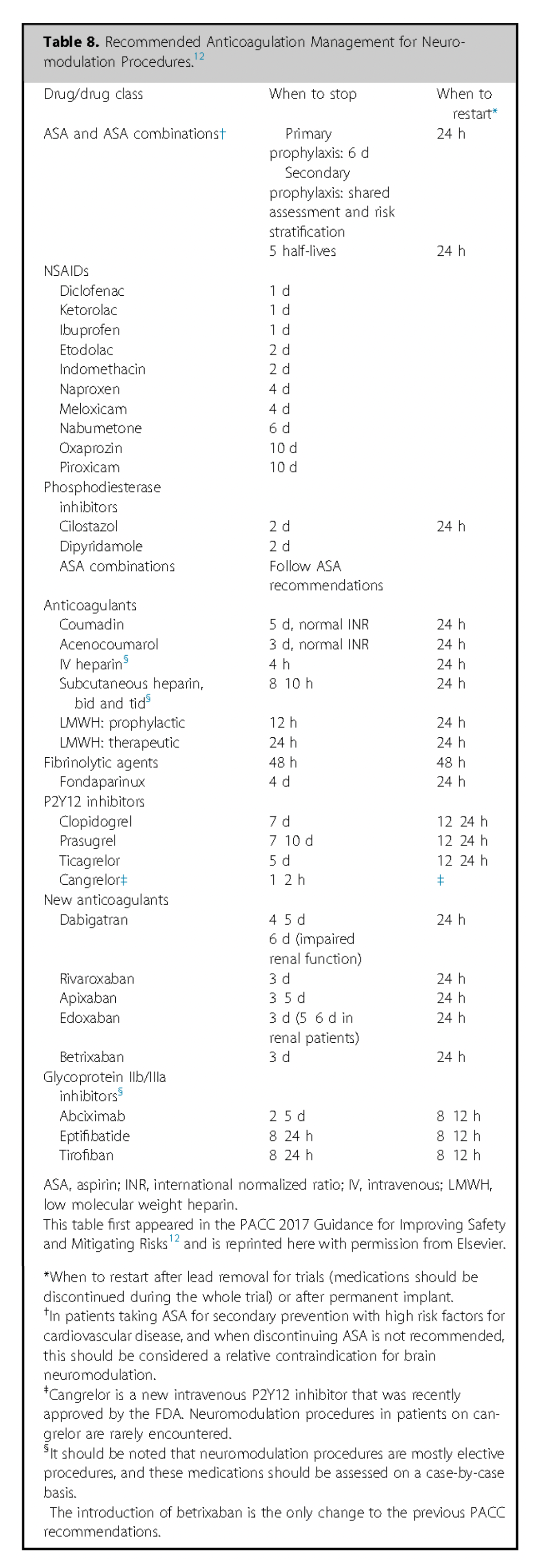
For antibiotic prophylaxis cephalosporins are preferred. In cases of stated penicillin allergy, consider alternatives like clindamycin or vancomycin; however specific testing for penicillin or preoperative test-dose administration may clarify sensitivity and allow for use of the superior antibiotic 233, 234.
Preoperative chlorhexidine baths and nasal mupirocin may be warranted12, 102.
Patient Education and Social Support
Educate the patient about the procedure, the implanted device, and the medications to be used.
Ensure the patient has adequate social support for postoperative recovery and follow-up visits.
Surgical Planning
On the day of surgery, mark incision sites to avoid impinging on costal margin or iliac crest, while the patient is seated, to avoid complications.
Review preoperative imaging, particularly cross-sectional spine imaging (MRI or CT), to assess anatomical considerations.
Consensus Point 29: Careful preoperative assessment of existing comorbidities, candidacy for anesthesia, medical optimization, preoperative imaging review, and surgical planning are crucial. USPSTF grade A; level of certainty high; level of evidence IA.
Intraoperative Considerations
Surgeons should have completed a minimum number of supervised implantations to ensure proficiency 235,10.
Avoid general anesthesia or heavy sedation during spinal access unless patient anxiety or pain necessitates it12.
Discuss positioning with the OR staff and anesthesia provider; ensure proper support and padding for lateral decubitus or prone positions.
Ensure proper skin preparation with alcohol-based antiseptic agent and allow adequate drying time before draping.
Make a precise incision and use electrocautery to minimize blood loss.
Review preoperative imaging to identify the conus medullaris and use fluoroscopy to optimize paramedian needle entry site, with L2-3 or L3-4 the preferred levels for dural entry.
Observe free-flowing cerebrospinal fluid (CSF) before catheter passage; use AP and lateral views to guide passage along dorsal aspect of intrathecal sac and do not retract catheter to avoid shearing forces on the catheter.
Consensus Point 30: PACC recommends dorsal catheter location with catheter tip at the dermatomal level concurrent with pain if anatomically indicated. USPSTF grade B; level of certainty moderate: level of evidence II
After ensuring free CSF flow anchor the catheter following manufacturer’s recommendations securely to reduce migration risk. Non-absorbable sutures are preferred.
The pump should be prepared by trained personnel in the sterile operative suite with transport medium removed and replaced with verified prepared drug, or preservative free saline if other desired intrathecal solution will be added later in the office or clinic.
All wounds should be thoroughly irrigated with sterile saline to reduce infection risk102, and adding antibiotics to the solution has not shown added benefit for clean surgeries.
Gentle tissue handling with appropriate instruments is crucial for optimal results.
Wounds should be closed in layers to minimize dead space using absorbable sutures. Options for skin closure include subcuticular sutures, surgical glue, or staples. Wounds should be covered with an occlusive dressing for 24–48 hours, with silver-impregnated dressings considered for high-risk patients.
A two-person verification protocol for pump programming post-surgery is recommended, with documentation provided to the patient.
Short-term postoperative antibiotics (not exceeding 24 hours) are recommended.
Reprinted from Neuromodulation: Technology at the Neural Interface, Vol 27 / Issue 7, Timothy R Deer, Salim M Hayek, Jay S Grider, Jonathan M Hagedorn, Gladstone C McDowell 2nd Philip Kim, Denis Dupoiron, Vasudha Goel, Rui Duarte, Julie G Pilitsis, Michael S Leong, Jose De Andrés, Christophe Perruchoud, Harry Sukumaran, Alaa Abd-Elsayed, Michael Saulino, Dennis Patin, Lawrence R Poree, Natalie Strand, Karina Gritsenko, Jill A Osborn, Ivano Dones, Anjum Bux, Jay M Shah, Brad L Lindsey, Erik Shaw, Tony L Yaksh, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain, Pages 1107-1139., Copyright (2024), with permission from the International Neuromodulation Society.
Postoperative Inpatient Management for IDDS Patients
If the patient will be admitted as an inpatient a thorough history and physical examination should be conducted, focusing on the pump interrogation, imaging if necessary, and pain management settings.
Adjustments to pump infusion settings can be made for pain management in a monitored setting.
Device pocket discomfort is expected postoperatively and can last up to 12 weeks. Analgesics may be used, but if pain persists, consider causes such as infection, seroma, hematoma, or device movement.
For deep anchoring or sustained neuropathic pain, medications like neuropathic pain agents or lidocaine patches may be effective.
Optimal surgical technique and snug fitting of the pump in the pocket are crucial to minimize movement. Sutures can help anchor the device and if movement leads to complications, corrective surgery may be necessary.
Small pocket or lumbar wound fluid accumulation is common, but large amounts may indicate seroma. Manage with pressure, aspiration, and potentially surgical drainage. If pain control with escalating dose fails, suspect an Intrathecal Catheter-Tip Granuloma (ICTG) 12, 149. MRI is the gold standard for diagnosis, with CT myelography as an alternative if MRI is contraindicated 240, 12.
Battery life for peristaltic pumps is approximately seven years. Off-label medications may increase pump failure risk; consider earlier replacement241.
If changing pump manufacturers, reduce the drug dose by approximately 10% during the transition.
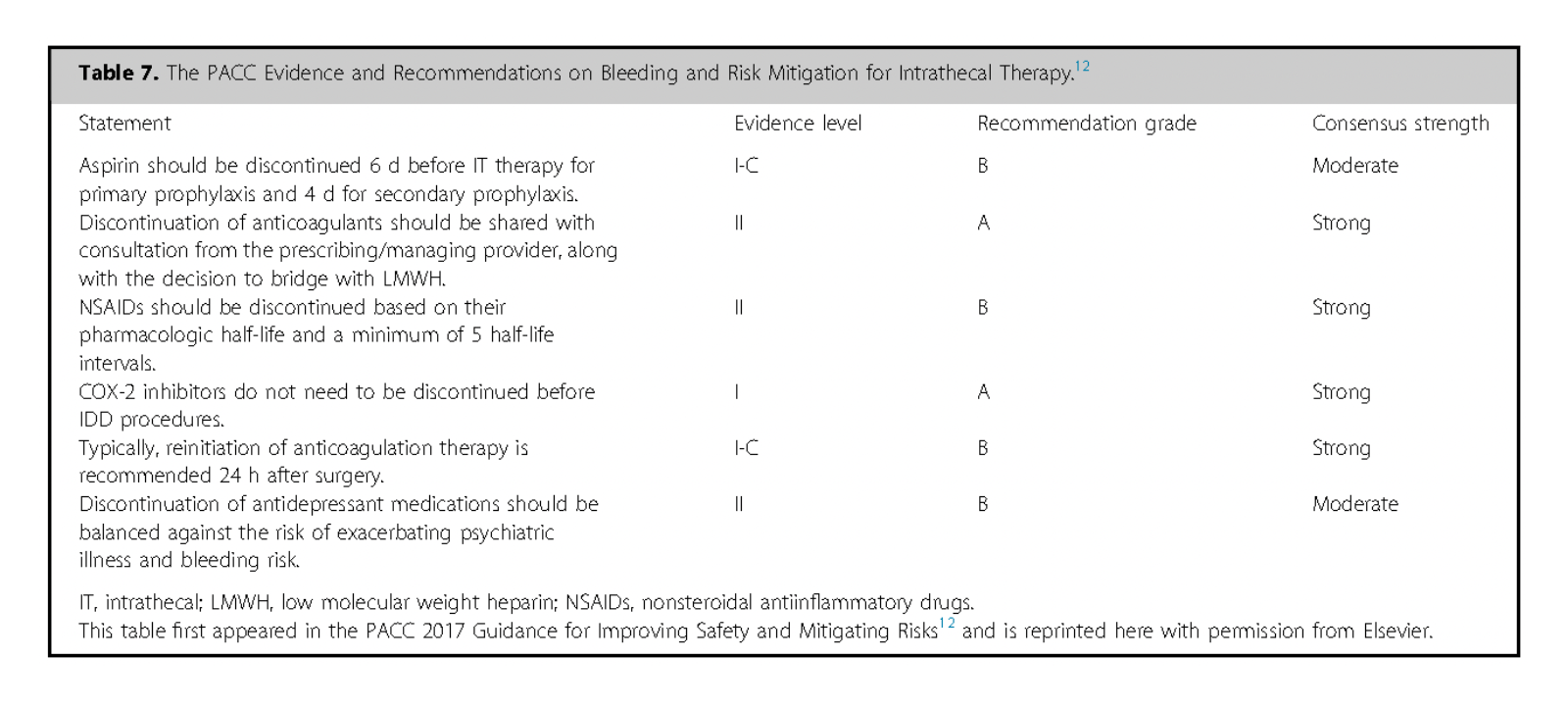
Perioperative anticoagulation Issues necessitate preoperative consultations with prescribing physicians and Oncologists are essential to assess bleeding risks, particularly for patients on anticoagulants or undergoing chemotherapy.
Placement of spinal catheters is high risk; pump placement is intermediate risk. Plan for mechanical and pharmacologic prophylaxis as needed 242.
Reprinted from Neuromodulation: Technology at the Neural Interface, Vol 27 / Issue 7, Timothy R Deer, Salim M Hayek, Jay S Grider, Jonathan M Hagedorn, Gladstone C McDowell 2nd Philip Kim, Denis Dupoiron, Vasudha Goel, Rui Duarte, Julie G Pilitsis, Michael S Leong, Jose De Andrés, Christophe Perruchoud, Harry Sukumaran, Alaa Abd-Elsayed, Michael Saulino, Dennis Patin, Lawrence R Poree, Natalie Strand, Karina Gritsenko, Jill A Osborn, Ivano Dones, Anjum Bux, Jay M Shah, Brad L Lindsey, Erik Shaw, Tony L Yaksh, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain, Pages 1107-1139., Copyright (2024), with permission from the International Neuromodulation Society.
Reprinted from Neuromodulation: Technology at the Neural Interface, Vol 27 / Issue 7, Timothy R Deer, Salim M Hayek, Jay S Grider, Jonathan M Hagedorn, Gladstone C McDowell 2nd Philip Kim, Denis Dupoiron, Vasudha Goel, Rui Duarte, Julie G Pilitsis, Michael S Leong, Jose De Andrés, Christophe Perruchoud, Harry Sukumaran, Alaa Abd-Elsayed, Michael Saulino, Dennis Patin, Lawrence R Poree, Natalie Strand, Karina Gritsenko, Jill A Osborn, Ivano Dones, Anjum Bux, Jay M Shah, Brad L Lindsey, Erik Shaw, Tony L Yaksh, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain, Pages 1107-1139., Copyright (2024), with permission from the International Neuromodulation Society.
Spinal Hematoma
The incidence of epidural hematoma in SCS implantations is approximately 0.75% 10, 243.
There is not much in the way of specific literature on this in pumps, but with cardiac pacemaker/defibrillator implantation the risk is higher in patients on anticoagulation therapy, with an estimated 2.2% incidence in this group 244. Patients who discontinued warfarin preoperatively with heparin-bridging and resumed it post-surgery experienced significantly fewer complications 245. Anticoagulation for device implantation is considered high-risk, and healthcare providers are advised to follow specific guidelines for high-risk procedures .
Issues such as migration, breakage, occlusion, and disconnection may be observed with catheter placements.
If revision surgery is needed careful steps should be taken, including avoiding cerebrospinal fluid (CSF) leakage by either removing the old catheter or ensuring the new one’s correct placement. Proper catheter handling is essential to prevent overdose or infection post-surgery.
Consensus Point 31: Recent publications classify anticoagulation risk on the basis of invasiveness. Intrathecal catheter placement is considered high risk on anticoagulation, and reference to society guidelines for catheter high-risk procedures should be followed. USPSTF grade A; level of certainty high; level of evidence II.
Incidence of Complications and Revision Rates
Complications in IDDS range from 15% to 40%, with an increase in incidence over long-term follow-up 204, 246, 194. Most adverse events are linked to catheter issues.
In a review of 1,362 implants, 558 complications were reported, with 66% related to the catheter, 27% to the surgical procedure, and 7% to the pump 247. Common catheter issues include migration, breakage, occlusion, and disconnection, with laceration occurring more often with midline placements. When catheter fragments are asymptomatic, removal is generally not attempted. If catheter malfunction is detected, intrathecal therapy should be minimized and reinitiated as necessary. In cases of newly discovered catheter disruption during surgery, patients should be monitored for at least 24 hours post-revision to manage the risk of overdose.
Consensus Point 32: When an intrathecal catheter becomes obsolete, various management techniques have been used and include 1) removing the old catheter with careful attention to CSF leak and 2) leaving the current catheter in situ and ensuring the complete obstruction of the lumen preventing CSF leak. At present, there is no evidence to favor one approach over the other. USPSTF grade A; level of certainty moderate; level of evidence II.

More effective management of intrathecal drug delivery.
© Copyright 2025. All rights reserved.

More effective management of intrathecal drug delivery devices.
© Copyright 2025. All rights reserved.
