Review of IT Medications
2024 Cancer PAIN
Review of IT Medications
2024 CANCER PAIN
Understanding the pharmacology of intrathecal (IT) agents is crucial for the safe and effective use of intrathecal drug delivery (IDD) systems. Opioids are the most commonly used class of medications in IDD, particularly for chronic pain management. There is considerable variation in clinical practice when it comes to IT medication delivery. Some centers perform pre-implantation trials routinely66, while others may not. This variability often stems from the limited availability of high-quality randomized controlled trials (RCTs) that provide definitive guidance on IT therapy protocols.
Using opioid dose-conversion calculations to determine IT opioid trial doses is not recommended67. The complexities arise due to the differences in pharmacology between systemically and intrathecally delivered opioids, as well as changes in systemic opioid use prior to the trial (e.g., oral tapering). These factors make it difficult to derive a standard conversion ratio. A cautious and individualized approach is recommended when determining IT doses for trials and subsequent long-term therapy.
Morphine
IT morphine is approved for continuous intrathecal use in treating chronic pain of both cancer and non-cancer origins68 and is the most widely used IT medication and considered as a first-line medication27. It can be delivered through intermittent bolus, constant infusion, or a combined regimen. Delivering morphine directly to the subarachnoid space increases its potency compared to oral (PO) or intravenous (IV) routes. This approach bypasses first-pass metabolism and the blood-brain barrier, allowing medication to act directly on the dorsal horn of the spinal cord. IT administration also reduces the systemic side effects often seen with oral or systemic opioid therapy, making it an option for patients who experience intolerable side effects from oral opioids.
Morphine primarily acts as a mu opioid receptor agonist at various central sites, including the spinal cord’s substantia gelatinosa and dorsal root ganglia69,70. Activation of these receptors presynaptically blocks calcium channels, preventing the release of neurotransmitters and postsynaptically increases potassium conductance, leading to hyperpolarization and reduced excitability of nociceptive neurons71,72.
Onset of action is 5 to 10 minutes after administration with duration of effect up to 20 hours. It is metabolized via hepatic glucuronidation to morphine-3-glucuronide, which is pharmacologically inactive. Excretion is primarily renal, with a small amount excreted unchanged13.
Common side effects include respiratory depression, myoclonus, nausea, vomiting, constipation, peripheral edema, pruritis, hypotension, bradycardia, urinary retention, and dependency. These adverse events are typical of opioids.
IT morphine administration can cause respiratory depression in two phases: an early phase (1-3 hours) and a late phase (6-12 hours) post-administration. Exercise extreme caution during initiation or restarting IT therapy and with rapid titration and avoid use of concomitant CNS depressants28,68.
Long-term use can disrupt hormone levels and result in complications such as adrenal insufficiency.
IT morphine lowers the seizure threshold and should be used cautiously in patients predisposed to seizures and abrupt cessation may result in withdrawal syndrome68.
Chronic infusion of morphine can lead to granuloma formation at the catheter tip, causing local mass effects and spinal cord compression73,74,75,76.
The proposed mechanism is that granulomas develop due to morphine’s activation of the Mas-related G-protein coupled receptor (MRGPR)79, which induces mast cell degranulation and fibroblast proliferation78,80. The result is the formation of space-occupying lesions near the catheter tip77;however other opioids that do not appear to activate mast cell degranulation have resulted in pericatheter mass formation81. The incidence of granuloma formation may have decreased over the years potentially due to the guideline recommendations of previous PACC publications30.
Symptoms include worsening back pain, new-onset lower extremity pain, sensory deficits, and myelopathic symptoms82.
MRI with/without or CT myelogram imaging are used to diagnose granulomas. Management may include cessation of IT therapy74, reduction in drug concentrations, catheter relocation, or switching to an alternative IT medication82.
Long-term studies have shown that IT morphine effectively reduces pain severity and decreases the need for systemic opioids in both cancer and non-cancer patients31.
A small retrospective study involving nine cancer patients at end-of-life showed all patients being pain-free after initiation of IT therapy86. The study reported fewer complications attributable to morphine compared to clonidine and bupivacaine.
Hydromorphone
A primary mu opioid agonist and can be used as a first- or second-line treatment in intrathecal (IT) therapy27,87. It is more water-soluble than morphine, which allows for the preparation of higher concentration solutions that require lower infusion volumes, making it advantageous in certain clinical scenarios87.
Preclinical and clinical studies have shown that intrathecal hydromorphone, particularly at higher concentrations, can lead to the formation of granulomas at the catheter tip74,88. The incidence of granulomas in patients receiving IT hydromorphone is reported to be around 8.7%, even at lower concentrations84.
Granulomas can cause complications such as local mass effects, spinal cord compression, and neurological symptoms, necessitating careful monitoring and potential intervention, such as dose reduction, catheter repositioning, or changing to a different opioid.
A large randomized, single-blind, non-inferiority trial was conducted to compare the efficacy of IT hydromorphone with IT morphine in patients with refractory cancer pain (n=233)18. Participants had either inadequate pain relief with >200 mg oral morphine equivalent (OME) daily doses or intolerable side effects from systemic opioids. IT infusion rates were calculated using a ratio of 1:300 for intrathecal:oral opioid equivalence, and hydromorphone:morphine equivalence was set at 0.15:1.
Both hydromorphone and morphine groups achieved similar pain relief, with 70.2% of hydromorphone patients and 70.5% of morphine patients achieving at least 50% pain reduction. Hydromorphone required significantly fewer bolus doses for breakthrough pain management compared to morphine. Over time, IT morphine doses increased, whereas IT hydromorphone doses decreased, suggesting a more stable dosing requirement with hydromorphone.
The safety profiles of both opioids were comparable, with constipation and nausea being the most common adverse events. Given its lower dose requirements and stable pain control, hydromorphone may be preferred for patients with refractory cancer pain.
Due to its higher solubility and lower infusion volume requirements, hydromorphone may be more suitable for patients who require high-concentration IT medications or those who experience complications from morphine18.
Fentanyl
A highly lipophilic mu opioid agonist, which allows it to cross cell membranes rapidly and act on mu opioid receptors within the central nervous system, providing potent analgesia.
Intrathecal (IT) fentanyl is generally not associated with granuloma formation. However, rare case reports have described granuloma development at high fentanyl infusion concentrations89,90. This suggests that while fentanyl carries a lower granuloma risk compared to other opioids like morphine or hydromorphone, it is not entirely without risk.
There are no direct studies evaluating the use of IT fentanyl specifically for oncologic pain. However, its use has been studied in other chronic pain conditions, providing insights into its clinical application.A retrospective study reviewed the use of IT fentanyl in patients with lumbar postlaminectomy syndrome. The study involved 58 patients who received either: IT Hydromorphone/Bupivacaine (30 patients), or IT Fentanyl/Bupivacaine (28 patients).
The patients in the IT fentanyl group had a lower rate of IT opioid dose escalation compared to those in the hydromorphone group. Both groups achieved similar reductions in pain scores. There were no cases of granuloma formation observed in either group after at least 2 years of follow-up.
The study authors suggest that IT fentanyl may lead to a lower rate of opioid dose escalation and could potentially be a safer option than hydromorphone, though this has not yet been confirmed in randomized controlled trials.
Sufentanil
A highly lipophilic mu opioid agonist, similar to fentanyl, with a rapid onset and potent analgesic properties. Its lipophilicity enables it to cross cell membranes quickly and exert its effects on mu opioid receptors in the central nervous system. Intrathecal sufentanil is primarily used when other opioids have failed to provide adequate analgesia, often as a second- or third-line treatment option30.
Intrathecal (IT) sufentanil is generally not associated with granuloma formation, though clinical experience is more limited compared to other opioids like morphine and hydromorphone. As with fentanyl, rare case reports suggest that granuloma formation is possible at high drug concentrations, but the overall risk is considered low30,81,92.
No new studies have been identified evaluating the efficacy of IT sufentanil specifically for chronic or oncologic pain management
While sufentanil has a favorable profile in terms of granuloma risk, its use should still be monitored closely, especially at high concentrations. Potential complications include typical opioid-related side effects like respiratory depression, nausea, and sedation.
Bupivacaine
An amide-type local anesthetic frequently used off-label in the United States for intrathecal (IT) therapy94,95. Its primary mechanism of action involves the inactivation of membrane-bound voltage-gated sodium channels on neurons96, which are critical for the transmission of nociceptive (pain) signals97.
Bupivacaine’s interaction extends beyond sodium channels; it also affects other membrane channels like the NMDA receptor, altering neuronal excitability and contributing to its analgesic properties98. It has a preferential effect on the nerve rootlets (fila radicularia) due to its high lipid solubility, which allows it to penetrate neuronal membranes effectively.
When delivered at low concentrations, bupivacaine alters sensory processing without affecting motor function, making it useful in pain management.
It may work synergistically with other IT agents (e.g., opioids), providing enhanced pain relief.
Bupivacaine does not exhibit tachyphylaxis (diminished response to a drug following repeated doses) in patients with neuropathic or somatic pain, making it suitable for long-term use.
The common adverse effects can include urinary incontinence, numbness, weakness, respiratory depression, and sympathectomy-induced hypotension.
There are case reports of permanent neurological damage, including toxic myelitis and arachnoiditis, presenting as progressive weakness and burning dysesthesias (abnormal sensations) in the lower extremities99,100. These complications are rare and may be associated with high drug concentrations as described in animal studies and case reports.
Patients do not typically experience withdrawal symptoms if IT bupivacaine is abruptly discontinued due to catheter or pump malfunction.
In patients with existing neuraxial disease or treatment-related injury (e.g., radiation neuritis or leptomeningeal disease), bupivacaine can increase the risk of adverse neurological effects.
In patients with high risk of neurological injury from their oncologic disease , the use of bupivacaine should be considered cautiously to prevent misattribution of evolving neurologic symptoms to the local anesthetic effect.
Since the 2017 PACC guidelines publication a retrospective study of 173 patients with stage IV cancer who received intrathecal drug delivery systems (IDDS) for refractory pain showed that 63.6% were treated with a combination of opioid and bupivacaine.
The combination therapy was associated with a significant reduction in systemic opioid use within the first 30 days post-implantation, with many patients completely stopping systemic opioid use19.
Another retrospective study of 50 cancer pain patients reported a median reduction in oral morphine equivalents (OME) from 503 mg preoperatively to 105 mg at 6 months postoperatively using IT morphine and bupivacaine combination therapy suggesting a synergistic benefit when bupivacaine is combined with IT opioids, allowing for lower opioid doses and potentially reducing opioid-related side effects101.
There is currently no consensus on starting or maximal doses of IT bupivacaine, and no prospective studies have evaluated its efficacy as a sole IT agent
Baclofen
Baclofen’s precise mechanism of action is not fully understood. However, it is known to exert its effects by acting as a gamma-aminobutyric acid (GABA)-B receptor agonist and limited literature suggest effective monotherapy for neuropathic pain102.
Baclofen decreases the release of excitatory neurotransmitters at presynaptic GABA-B receptors, which leads to reduced neuronal excitability.
At postsynaptic GABA-B receptors, baclofen induces hyperpolarization (a state where the neuron becomes less likely to fire) by increasing potassium conductance and inhibiting calcium channels. This results in diminished synaptic transmission and provides a potent muscle relaxant and antispasticity effect.
While uncommon, there is at least one case report suggesting the formation of inflammatory masses (granulomas) in patients receiving IT baclofen for spasticity management104.
Abrupt discontinuation of IT baclofen can precipitate life-threatening withdrawal symptoms, which may manifest as early as 24 hours after discontinuation or occur gradually105. These include respiratory failure, tachycardia, tachypnea, fever, seizures, altered mental status, and cardiovascular instability. The definitive treatment for baclofen withdrawal is the resumption of IT therapy. High oral doses of baclofen and benzodiazepines can be used as temporary measures.
The SISTERS trial, a large and recent multicenter randomized controlled trial (RCT), evaluated the efficacy and safety of IT baclofen (ITB) therapy versus conventional medical management (CMM) for poststroke spasticity (PSS)114.
The ITB therapy group had significantly greater improvements in spastic hypertonia (increased muscle tone) and muscle function compared to the CMM group.
There are currently no direct studies evaluating IT baclofen specifically for use in oncologic pain management. The existing data is largely focused on spasticity management, particularly in neurological conditions such as multiple sclerosis and poststroke spasticity.
Clonidine
Works by selectively agonizing alpha-2 adrenergic receptors in the dorsal horn of the spinal cord116. At presynaptic receptors, clonidine reduces the release of excitatory neurotransmitters (e.g., glutamate and substance P) from nociceptive afferents, thereby decreasing pain signal transmission117. At postsynaptic receptors, clonidine induces hyperpolarization, increasing potassium conductance and inhibiting calcium channels, which results in reduced neuronal excitability and inhibition of nociceptive pathways118.
While primarily used as an antihypertensive agent, clonidine has demonstrated effectiveness in treating both acute and chronic pain, particularly neuropathic pain, when administered intrathecally (IT). It is often used in combination with opioids for pain relief in patients who have not responded adequately to opioid monotherapy.
Clonidine is not currently FDA-approved for IT administration, though a review of more than 25 studies supports its use in treating chronic pain with a neuropathic component115.
Clonidine can cause severe hypotension, particularly at the onset of IT therapy. Hypotension occurs in approximately 25% of patients upon initiation119.
Because of its impact on sympathetic tone, clonidine can reduce peripheral vascular resistance, heart rate, and blood pressure.
Abrupt withdrawal of IT clonidine may lead to serious complications, including agitation,headache,hypertensive crisis and even hemodynamic instability39.
In cases of withdrawal-induced hypertension, intravenous phentolamine or clonidine can be used to manage the condition.
A review by Kumar et al. summarized 30 clinical trials using clonidine as a sole agent or in combination with other drugs for chronic pain management115. While clonidine showed some benefit, its utility was limited, and selective use is recommended.
A study by Hassenbusch et al. with 31 patients, clonidine monotherapy provided long-term pain relief in 59% of patients who had previously failed IT opioid therapy119. Similar findings were reported by Ackerman et al., with clonidine providing pain relief in 70% of patients120.
Siddal et al. conducted a double-blind randomized controlled trial (RCT) in 15 patients, demonstrating that a combination of morphine and clonidine produced greater pain relief than either medication used alone121.
Rudich et al. showed that clonidine is stable when combined with hydromorphone for long-term use in IT pumps122.
Due to its cardiovascular effects, the risk-benefit ratio of clonidine should be carefully evaluated before initiating IT therapy, particularly in patients with pre-existing cardiac conditions.
Ziconotide
Ziconotide is one of the three agents approved by the FDA for intrathecal (IT) use and one of two approved for IT treatment of chronic pain. It is a non-opioid, non-NSAID analgesic used for managing severe chronic neuropathic and nociceptive pain.
Ziconotide works by reversibly blocking N-type calcium channels in the dorsal horn of the spinal cord125. It was discovered from the venom of cone snails and is effective in opioid-tolerant patients due to its unique mechanism, which is independent of the mu-opioid receptor126.It has been recommended as first-line therapy for both non-cancer and cancer pain31.
In terms of side effect profile ziconotide is generally well-tolerated and is not associated with catheter tip granuloma formation, respiratory depression, withdrawal syndrome and is not neurotoxic at concentrations used in clinical practice127,128,129.
Common adverse effects include nausea, confusion, dizziness, hallucinations, and urinary retention, often related to the rate of titration rather than the dose itself130. Slow and low-dose titration starting at 1.2 µg/day over a 6-month period can help minimize these side effects130,131.
Several studies have shown that combining ziconotide with IT opioids can provide effective pain relief, with minimal adverse effects when titrated slowly. A single-center retrospective observational study demonstrated that adding ziconotide to an IT opioid regimen in patients with incomplete pain relief was safe and effective132.
Staats et al 133and Rauck14 RCTs demonstrated the efficacy of ziconotide in short-term follow-up (weeks), particularly when used as part of a combination regimen.
The multicenter PRIZM registry study interim report by Deer et al 130 and subsequent final report by McDowell et al 134 focused on low-dose ziconotide strategies for pain outcomes. While these studies showed promising results, high dropout rates due to ziconotide-related adverse effects (AEs) indicate a need for cautious dosing and monitoring.
A 2015 Prospective Trial (n=98): Found that 42% of patients received a combination of opioid and bupivacaine, 40% received opioid monotherapy, and 9% received opioid, bupivacaine, and ziconotide20. Only 7% were on ziconotide monotherapy.
In 2020 a retrospective review at a large cancer center(n=173) showed a reduction in systemic opioid use by 94% at 30 days post-implant, with 82.6% of patients discontinuing systemic opioids completely19.
A retrospective pancreatic cancer pain study (2018, n=93) where all patients received combination therapy showed that 90% received low-dose ziconotide (0.5 ± 0.2 µg/day) alongside morphine and ropivacaine16.
Due to its unique mechanism, ziconotide often complements opioids and local anesthetics in cancer pain management. Combining ziconotide and morphine has shown synergistic analgesic effects in both animal models and human studies, allowing for effective pain control with lower doses of each medication.
PACC Guidelines
Previous guidelines emphasized ziconotide as a first-line agent in cancer pain, but its clinical use has been more variable. The updated guidelines suggest using ziconotide for patients with limited life expectancy (Table 3) or in cases where rapid titration is not essential (Table 4). Regimen selection should consider pain characteristics, catheter tip location, and individual patient factors and these new designations are an update to the 2017 PACC recommendations31.
Table 3. Cancer Pain Treatment in a Patient with Limited Life Expectancy. In a patient with limited life expectancy (less than 6 months) aggressive dose titration is implemented to obtain satisfactory analgesia. Regimen selection should also be based on pain characteristics, catheter tip location, and the individual drug factors presented in Table 5.
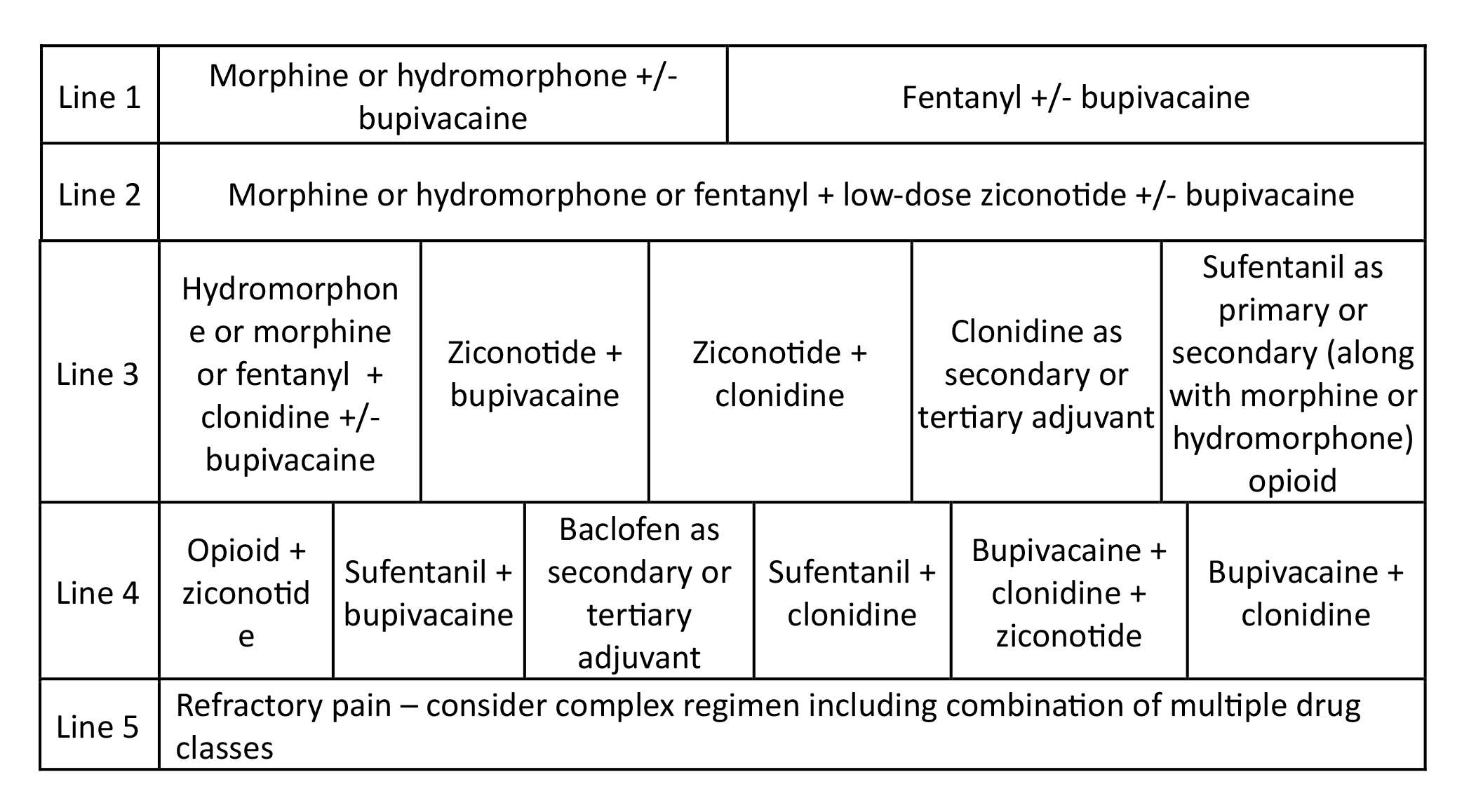
Reprinted from Neuromodulation: Technology at the Neural Interface, Timothy R Deer, Salim M Hayek, Jay S Grider, Jason E Pope, Shane E Brogan, Amitabh Gulati, Jonathan M Hagedorn, Natalie Strand, Jennifer Hah, Tony L Yaksh, Peter S Staats, Christophe Perruchoud, Nebojsa Nick Knezevic, Mark S Wallace, Julie G Pilitsis, Tim J Lamer, Eric Buchser, Vishal Varshney, Jill Osborn, Vasudha Goel, Brian A Simpson, Jose A Lopez, Denis Dupoiron, Michael F Saulino, Gladstone C McDowell 2nd , Fabian Piedimonte, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain, Pages No., Copyright (2024), with permission with permission from the International Neuromodulation Society.
Table 4. Chronic Cancer Pain Treatment with Favorable Prognosis (6 months to years). Emphasis is on attaining improvement in pain and function, while considering durability and safety of therapy for a long period. Regimen selection should also be based on the patient’s condition and individual drug factors presented in Table 5.
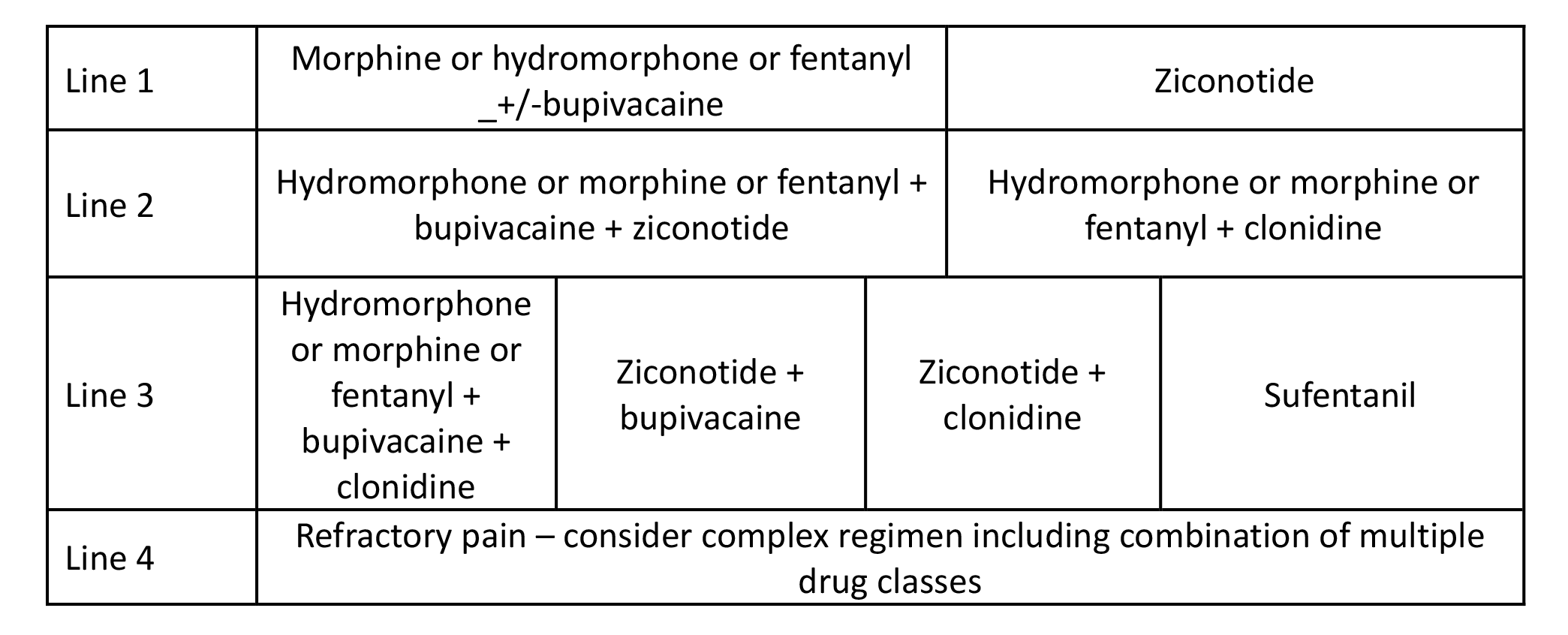
Reprinted from Neuromodulation: Technology at the Neural Interface, Timothy R Deer, Salim M Hayek, Jay S Grider, Jason E Pope, Shane E Brogan, Amitabh Gulati, Jonathan M Hagedorn, Natalie Strand, Jennifer Hah, Tony L Yaksh, Peter S Staats, Christophe Perruchoud, Nebojsa Nick Knezevic, Mark S Wallace, Julie G Pilitsis, Tim J Lamer, Eric Buchser, Vishal Varshney, Jill Osborn, Vasudha Goel, Brian A Simpson, Jose A Lopez, Denis Dupoiron, Michael F Saulino, Gladstone C McDowell 2nd , Fabian Piedimonte, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain, Pages No., Copyright (2024), with permission with permission from the International Neuromodulation Society.
Consensus Point 9. Ziconotide as a monotherapy may be considered lower in the treatment algorithm for recalcitrant cancer pain due to its need for slow titration. USPSTF Grade B; Level of certainty low; Quality of evidence II.
Table 5. Commonly Used Intrathecal Drugs in Cancer Pain. Practical Issues Driving Regimen Selection.
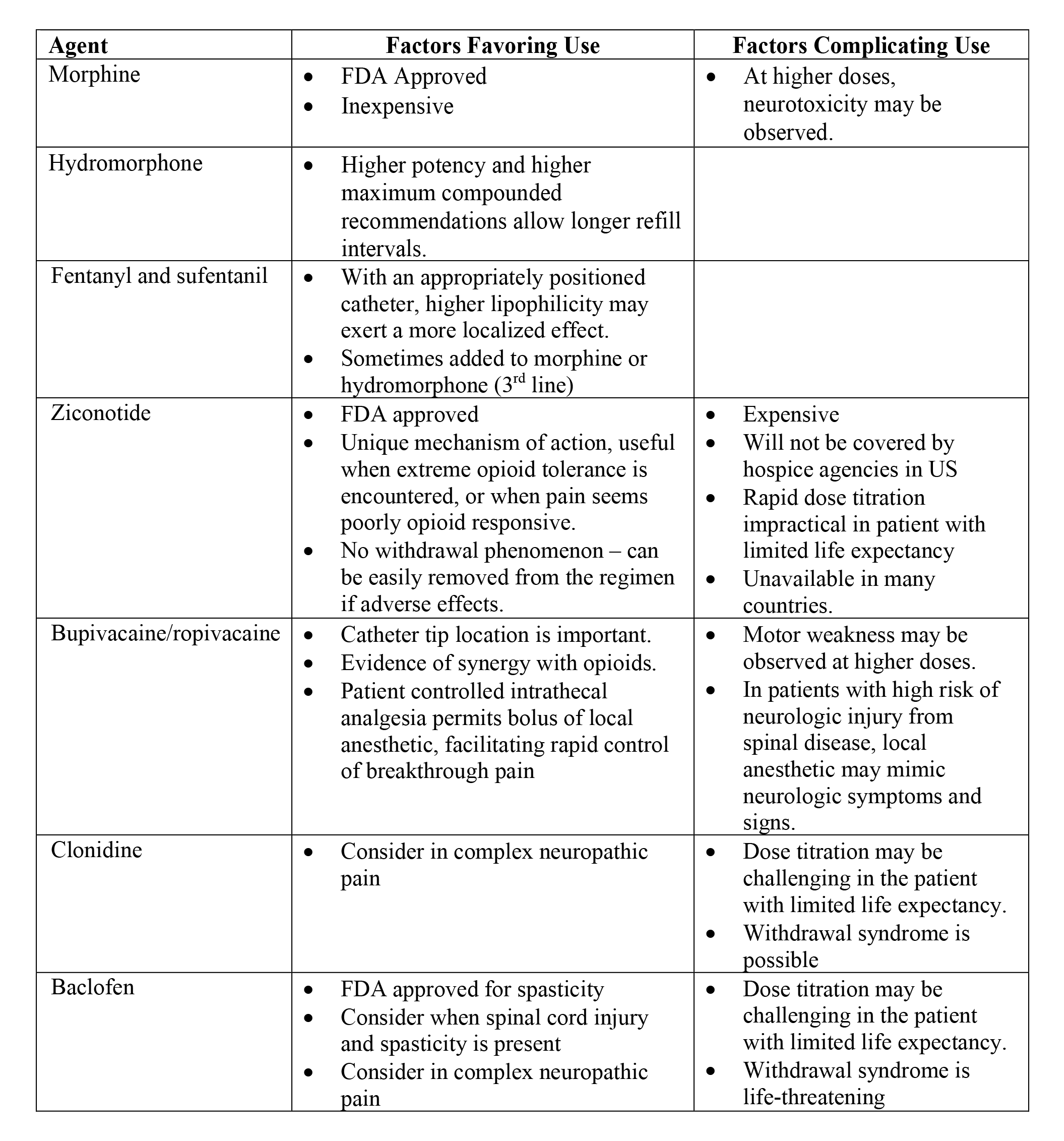
Reprinted from Neuromodulation: Technology at the Neural Interface, Timothy R Deer, Salim M Hayek, Jay S Grider, Jason E Pope, Shane E Brogan, Amitabh Gulati, Jonathan M Hagedorn, Natalie Strand, Jennifer Hah, Tony L Yaksh, Peter S Staats, Christophe Perruchoud, Nebojsa Nick Knezevic, Mark S Wallace, Julie G Pilitsis, Tim J Lamer, Eric Buchser, Vishal Varshney, Jill Osborn, Vasudha Goel, Brian A Simpson, Jose A Lopez, Denis Dupoiron, Michael F Saulino, Gladstone C McDowell 2nd , Fabian Piedimonte, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain, Pages No., Copyright (2024), with permission with permission from the International Neuromodulation Society.
Compounding Concepts
Compounding refers to mixing or combining ingredients, including altering commercial products, to create a final medication tailored for specific dosing or novel delivery. This process is regulated by regional authorities rather than national health bodies, and it is especially relevant in oncologic pain management due to the frequent use of compounded intrathecal (IT) medications. Standards for compounding are detailed in the United States Pharmacopeia (USP) Chapters 795 and 797, which address the quality control of both non-sterile and sterile preparations139. Clinicians are advised to understand the credentials and standards of compounding pharmacies when utilizing these medications.
Consensus Point 10: Given the strict definitions of what constitutes a compounded medication, most if not all IDD utilizes some degree of compounding. The PACC recommends that clinicians be aware of refill intervals and safety concerns when utilizing these medications. USPSTF Grade A; Level of certainty low; Quality of evidence 1-C.

More effective management of intrathecal drug delivery.
© Copyright 2025. All rights reserved.

More effective management of intrathecal drug delivery devices.
© Copyright 2025. All rights reserved.
