Updates on Implantation Issues
2024 Non Cancer PAIN
Updates on Implantation Issues
2024 NON CANCER PAIN
Catheter Location
The traditional understanding of CSF circulation has evolved to a more complex model involving oscillatory and pulsatile movements influenced by cardiac and respiratory cycles80,81. Drug distribution in the CSF is limited to a few centimeters from the administration site, making the placement of the catheter tip critical for therapy success60,64.
Pharmacokinetic Factors
- Hydrophilic Drugs: Display broader rostrocaudal movement in the CSF, resulting in lower systemic uptake and prolonged spinal analgesia65.
- Lipophilic Drugs: Have limited spread in the CSF and are quickly reabsorbed in systemic circulation65. Catheter placement near the targeted spinal segments is essential for these agents.
Bupivacaine has a limited distribution at low flow rates and should be administered with a dermatomal approach. For head and neck cancer pain or neuropathic facial pain, in combination with an opioid, catheter advancement as high as the C1 level has been reported84,85.
For lumbar and leg pain, a classic lumbar approach with catheter tip placement around T9-T10 can minimize migration risks. Retrograde placement may be effective for pelvic cancer pain83, and placement as high as C1 may be appropriate for head and neck pain.
Consensus Point 10: Intrathecal catheter placement should be targeted to the optimal vertebral level to cover spinal segments involved in the neuronal transmission of pain. USPSTF grade A; level of certainty moderate; evidence level IB.
CSF Dynamics and Infusion Strategies
Several studies failed to show any improvement in pain relief when increasing the continuous flow rate of drugs without changing the daily dose and switching from continuous to intermittent bolus administration has shown unpredictable results94,95,96. This suggests that merely adjusting pump programming does not sufficiently improve drug distribution. Cardiovascular changes, such as heart rate and stroke volume, impact drug distribution in ways that are difficult to predict in clinical settings62. A slower infusion rate may reduce cardiovascular effects on drug spread.
There are fewer reported complications when using bolus administration compared to continuous flow at comparable total daily doses97. However, bolus administration increases localized concentration (near the infusion site) but not overall spread.
Consensus Point 11: Switching modes of intrathecal catheter delivery from continuous to intermittent bolus may not produce improved outcomes. Impact on safety and efficacy both remain undetermined. USPSTF grade B; level of certainty moderate; evidence level IB.
Pump implantation sites
The standard practice involves placing the pump in the abdominal wall, as it avoids being in contact with clothing or daily movements98,99. Improper implantation, such as placing the pump too superficially, can result in complications like skin erosion, pump malfunction, or damage from daily activities. Special considerations are necessary for patients with a lower or higher body mass index (BMI), who may face difficulty with placement.
Several studies have explored alternative implantation sites for patients where abdominal placement is not feasible, such as in those with high BMI or complex anatomy. Alternative locations like the flank or anterior thigh have been examined, but these sites are associated with their own set of challenges, including body posture affecting pump positioning and difficulty in maintaining consistent placement100,101. It is also important to ensure that tubing is properly placed to avoid kinks or other issues, which could interfere with the functionality of the pump.
Consensus Point 12: The traditional implantation site for intrathecal pump placement remains in the abdominal wall. The PACC recognizes that the practice pattern is evolving and that for many practitioners, the buttock/posterior sites and other sites may be applicable and should be considered on a case-by-case basis. USPSTF grade B; level of certainty moderate; evidence level II.
Reducing Infection Rates
Infection prevention is a major concern during pump placement, catheterization, and post-operative care. The 2017 PACC10,12 and NACC102 recommendations include adhering to protocols for skin antisepsis, sterilization of the pump site, and maintaining strict cleanliness preoperatively, intraoperatively, and postoperatively. The use of preoperative screening protocols 105,107 and strict skin cleansing perioperatively is recommended. Evidence supports minimizing catheter manipulation and ensuring good wound care to reduce infection rates. Use of antibiotics before, during, and after the procedure, as well as the careful management of risk factors (like smoking or obesity), is advised to further reduce infection risks10,12.
Consensus Point 13: IDDS implanters should follow published guidelines on perioperative management of implanted devices and time medication administration optimally to prevent infection. USPSTF grade B; level of certainty moderate; evidence level IB.
Perioperative and Postoperative Management of the New Device
Perioperative education regarding surgical risk, wound and device management is crucial. Occlusive wound dressings, office follow up within 2 weeks, and judicious use of systemic opioids with IDD infusion are recommended.
Consensus Point 14: Clinicians should educate patients and their caregivers perioperatively about IDDS, the medications being administered, and potential adverse events. Clinicians also should be aware that the initial postimplant phase can be complicated by concomitant administration of systemic opioids. USPSTF grade B; level of certainty moderate; evidence level II.
Initial Pump Flow Settings
Initial flow rates are often low to ensure patient safety and avoid complications. A screening trial may be required and may provide information regarding efficacy and potential starting dose, with ziconotide being an exception. In the context of intrathecal pumps, doses may be measured in micrograms or milliliters per hour. The specific medication being administered (e.g., morphine, baclofen) greatly influences the initial flow rate. After the initial dose, flow rate is gradually increased or decreased depending on how well the drug alleviates symptoms and whether there are any side effects.
Continuous infusion is steady, low-dose continuous infusion, or microboluses to maintain consistent drug levels and is the most common intial infusion protocol. Slow flow rates with higher concentration morphine or hydromorphone may pose greater granuloma formation risk near the catheter tip8,10,12,113.
Flex dosing and multiple flow rates initially used for spasticity control with baclofen has been used for analgesia during the day or nocturnal delivery114,115,78.
Some pumps can deliver periodic single, or multiple, boluses without a continuous basal dose.
CSF velocity changes during drug bolus infusion depend on several factors, such as stroke volume, respiratory cycle, drug solution density, posture, and individual anatomical differences. These factors can alter drug dispersion and concentration116,117,118,119.
Computational models suggest that low flow rates can affect CSF flow dynamics. Injection with higher latency may lead to a greater drug concentration and volume of contamination in the CSF97,120.
Patient activated boluses allow patients to moderate their pain management by using devices (like handheld controllers or mobile apps) to administer additional doses when needed. These doses can account for 5-20% of the total drug volume.
The use of patient activated boluses with ziconotide therapy was reported in 2010 and has become an accepted method to titrate this medication121,122. The 2017 PACC recommendations based on the three pivotal ziconotide studies, and many years of post-FDA approval experience, suggest ziconotide dilution with preservative-free normal saline to allow dose initiation at 0.5 to 1.2 μg/d.
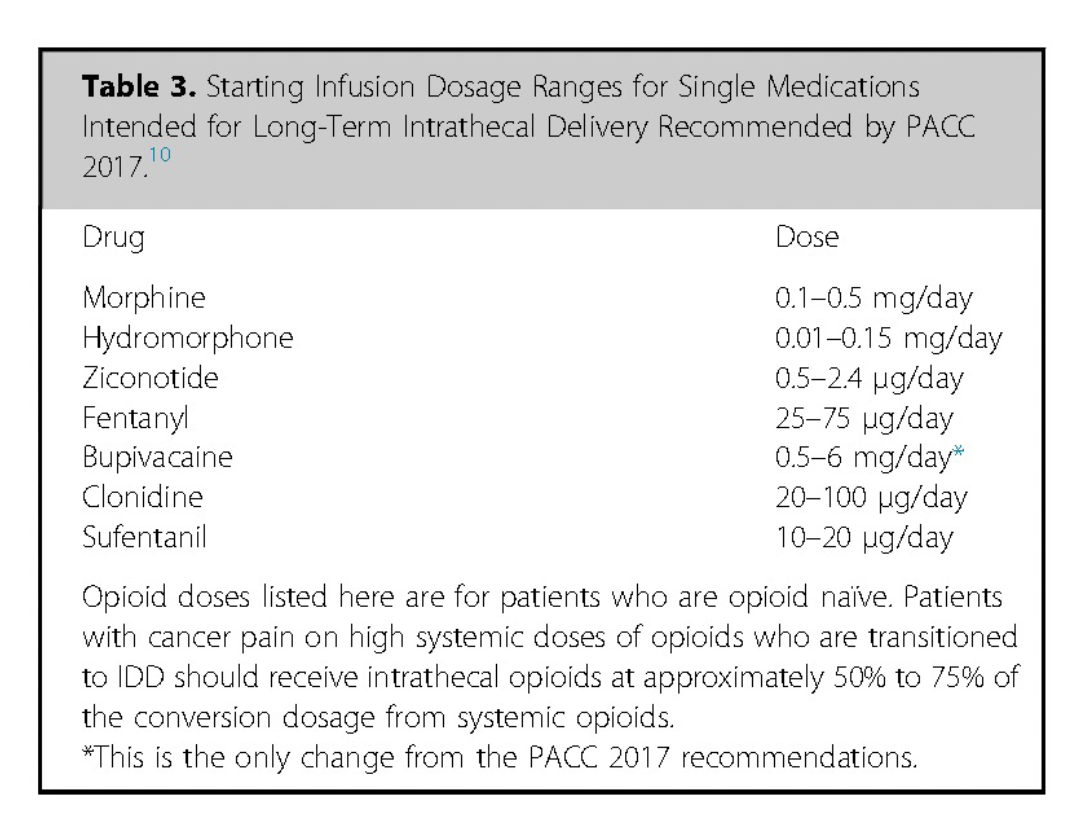
Reprinted from Neuromodulation: Technology at the Neural Interface, Vol 27 / Issue 7, Timothy R Deer, Salim M Hayek, Jay S Grider, Jonathan M Hagedorn, Gladstone C McDowell 2nd Philip Kim, Denis Dupoiron, Vasudha Goel, Rui Duarte, Julie G Pilitsis, Michael S Leong, Jose De Andrés, Christophe Perruchoud, Harry Sukumaran, Alaa Abd-Elsayed, Michael Saulino, Dennis Patin, Lawrence R Poree, Natalie Strand, Karina Gritsenko, Jill A Osborn, Ivano Dones, Anjum Bux, Jay M Shah, Brad L Lindsey, Erik Shaw, Tony L Yaksh, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain, Pages 1107-1139., Copyright (2024), with permission from the International Neuromodulation Society.
Early Postimplant Management Issues
Optimizing Pump Settings
After the implantation of an intrathecal drug delivery system (IDDS), it typically takes several weeks to optimize the pump’s dosing to achieve adequate symptom relief. This period involves regular follow-ups, usually weekly or biweekly, for adjustments if necessary. Doses are often increased by 10% to 25% increments every one to two weeks until the desired pain relief is achieved, provided that side effects remain tolerable. If symptom reduction is not observed after four to five dose increases, reevaluation and medication rotation should be considered. In patients with limited lifespan and poorly controlled cancer pain, a more aggressive titration regimen with shorter intervals may be necessary.
Two types of commercially available pumps are used for intrathecal medication delivery:
- Peristaltic Delivery System: Offers various flow settings, including continuous infusion over 24 hours, or flexible dosing (flex dosing). In flex dosing, a patient receives a slow bolus (e.g., 1000 μL/h) or a higher medication flow over a set period, in addition to a basal dose between boluses. Settings can be customized for continuous flow, continuous flow with patient-activated boluses, or flex dosing114.
- Valved System: Delivers medication in fixed 2-3 μL boluses repetively achieving a continuous delivery. It can be programmed for a constant daily dose, continuous delivery with patient-activated boluses, multiple rates, or periodic flow, ensuring precise medication delivery115.
Both systems enable patient-activated analgesia within programmable limits, with the Medtronic Patient Therapy Manager (PTM) and Flowonix Patient Therapy Controller (PTC) offering increased patient satisfaction and better pain control through self-administered boluses125.
Pumps can be programmed to optimize drug delivery by adjusting flow rates based on patient needs. The infusion can be continuous or patient-controlled, with the potential for delivering larger or more concentrated doses as needed114,115.
- Continuous Infusion: Delivers medication steadily over 24 hours.
- Flex Dosing: Provides a combination of basal medication delivery with a slow bolus at higher flow rates during certain periods.
- Periodic Flow: Uses programmed boluses at specific times with or without basal doses to ensure adequate drug distribution and effectiveness.
Common Issues in Transition to IDD
Managing expectations is crucial for long-term success with IDDS. Before implant and during follow-ups, patients should have realistic goals for pain relief and activities. Additionally, pre-implant weaning off oral opioids and benzodiazepines is often recommended, particularly in non-cancer pain patients, due to the increased risk of opioid-induced respiratory depression11.
Research suggests that patients who successfully wean off opioids before IDD implantation tend to achieve better long-term outcomes at lower intrathecal doses. Studies by Hamza et al. and Grider et al. have demonstrated that patients who reduced or eliminated systemic opioids before an intrathecal trial stabilized at lower intrathecal opioid doses post-implantation124,128.
For cancer pain patients, transitioning to IDD can be more complex, and weaning off opioids may be limited until after implant. In these cases, careful upward titration of the IDD system while reducing other opioid sources is necessary. See the PACC 2024 Cancer section of the app for more detail.
Consensus Point 15: Patients with chronic noncancer pain being considered for IDD with opioids should be weaned off other systemic opioids (or to the lowest possible dose) in consultation with their physicians, and preferably weaned off benzodiazepines if medically safe to do so. USPSTF grade B; level of certainty moderate; evidence level IB.
Wound Management
The most common postoperative complication is soreness around the pump site, which may develop into a seroma. If the wound is not infected, treatment with an abdominal binder is appropriate. However, symptoms like swelling, redness, drainage, or increased pain could indicate an infection and should be monitored closely and antibiotic treatment or surgical intervention considered as needed130,131.
For patients on anti-vascular endothelial growth factor (VEGF) drugs, the risk of wound dehiscence may be increased. Therefore, it is recommended to stop anti-VEGF treatment 15 to 20 days before implantation132.
Consensus Point 16: Careful attention should be paid to postoperative wound healing and wound care. In the event of a complication, the intervention required depends on the cause and severity of the complication. USPSTF grade A; level of certainty high; level of evidence II

More effective management of intrathecal drug delivery.
© Copyright 2025. All rights reserved.

More effective management of intrathecal drug delivery devices.
© Copyright 2025. All rights reserved.
