Delivery System-Related Complications
2024 Non Cancer PAIN
Delivery System-Related
Complications
2024 NON CANCER PAIN
Complications associated with IDD systems can be classified into three broad categories: perioperative procedure-related issues, medication-related adverse events, and delivery system issues. These complications can lead to morbidity, mortality, and drug-related issues such as underdosing or overdosing. Despite advancements in pump and catheter technology194, delivery system complications like pump and catheter malfunctions, and issues during refill or reprogramming, can still occur.
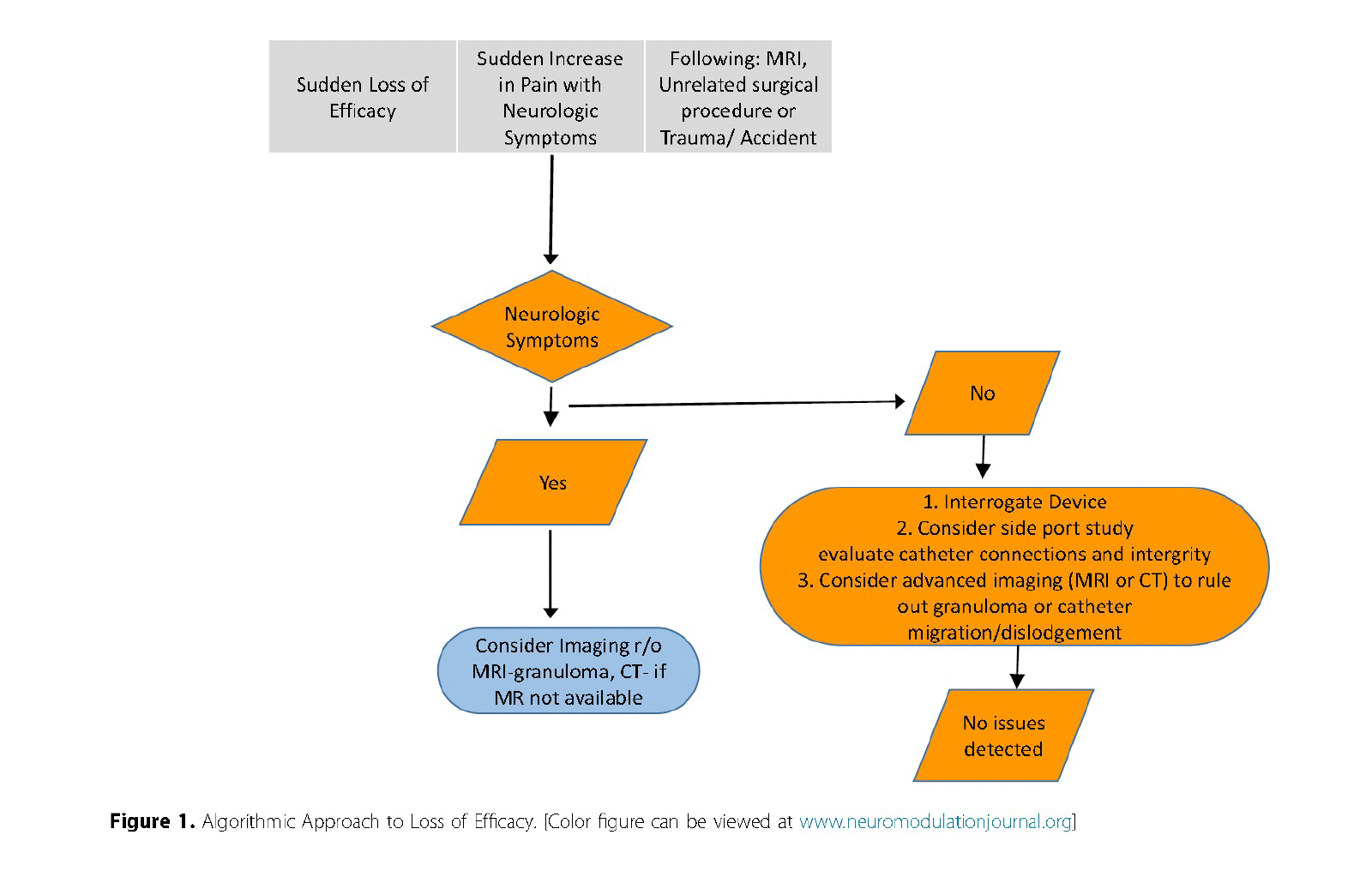
Reprinted from Neuromodulation: Technology at the Neural Interface, Vol 27 / Issue 7, Timothy R Deer, Salim M Hayek, Jay S Grider, Jonathan M Hagedorn, Gladstone C McDowell 2nd Philip Kim, Denis Dupoiron, Vasudha Goel, Rui Duarte, Julie G Pilitsis, Michael S Leong, Jose De Andrés, Christophe Perruchoud, Harry Sukumaran, Alaa Abd-Elsayed, Michael Saulino, Dennis Patin, Lawrence R Poree, Natalie Strand, Karina Gritsenko, Jill A Osborn, Ivano Dones, Anjum Bux, Jay M Shah, Brad L Lindsey, Erik Shaw, Tony L Yaksh, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain, Pages 1107-1139., Copyright (2024), with permission from the International Neuromodulation Society.
- Delivery System Malfunctions:
- Pump Malfunctions:
- Pump-related issues, though less frequent than catheter-related ones, can be life-threatening. The most common pump issues include catheter kink or occlusion (3.5%), motor stalls (0.9% incidence)55 and failures often associated with off-label medication use. For example, a case series involving 148 pumps showed a failure rate of 4.5% overall, 5.3% with off-label drug mixtures, and 2.8% with on-label morphine or ziconotide
- Recent FDA data (MAUDE) highlights motor stalls in 12.4% of the cases reported, with other common complications including infection and erosion (15.7%) and adverse medication reactions (11.8%)196.
- Exposure of pumps to high magnetic fields during MRI can cause temporary motor stalls, and the pump’s response varies depending on their mechanism. For example, peristaltic motors may stall during MRI but recover afterward, while valve-gated pumps may need to be emptied before MRI to prevent unintended drug release.
- Catheter Malfunctions:
- Catheter complications (e.g., kinks, occlusions, migration) are more prevalent than pump malfunctions. Approximately 14.4% of adverse events reported were catheter-related, with direct catheter damage accounting for 8.2%196.
- Investigating catheter function is crucial when faced with loss of analgesic efficacy. Algorithms typically involve a combination of plain radiography, aspiration from the catheter access port, and contrast studies.
- Techniques for Diagnosing Malfunctions:
- Pump Evaluation:
- Rotor studies (historically used to evaluate pump function) are rarely performed now. Audible alarms and event logs are now preferred to identify motor stalls or malfunctions.
- Catheter Evaluation:
- Routine screening for catheter function is recommended if there is clinical suspicion of a malfunction. Plain radiography is the initial step, but newer imaging modalities, such as CT myelography, can offer superior visualization, especially for catheters with limited radiographic visibility.
- Catheter access port aspiration is another critical technique, which, if unsuccessful, may necessitate surgical revision.
- Complications During Refills and Reprogramming:
- Refills and reprogramming pose risks, particularly “pocket fills,” where medication is inadvertently injected into subcutaneous tissue instead of the pump reservoir. This can cause rapid overdose or withdrawal symptoms217.
- The incidence of pocket fills is likely underreported but estimated to be around 1 in 10,000 refills218. They are particularly associated with pump hypermobility, deep implants, and inexperience of the operator. Strategies to minimize pocket fills include using fluoroscopic or ultrasound guidance during refills as supported by 20129 and 201712 PACC guidelines.
- Imaging Modalities and Safety Measures:
- Contrast Agents:
- In patients with iodine-based contrast allergies (IBCAs) gadolinium-based agents (GBCA) have been used as an alternative, but with caution193. GBCAs are not recommended for routine neuraxial procedures due to potential neurotoxicity.
- Imaging Techniques:
- Advances in CT myelography and 3D reconstructions can provide better precision in detecting catheter issues compared to traditional imaging techniques. Nuclear scintigraphy (Indium 111 DTPA) is another alternative but is limited by high cost, lack of FDA approval, and complexity.
Consensus Point 27: Catheter malfunction and malposition represent many of the issues related to IDD, and an algorithmic approach to evaluation is suggested as outlined in this section. USPSTF grade A; level of certainty high; level of evidence II.
Consensus Point 28: Ultrasound or fluoroscopic-guided access to the central fill port should be considered if there are concerns about body habitus, or device positioning that make template-guided approaches less certain. USPSTF grade B; level of certainty low; level of evidence II
- Emergency Preparedness and Protocols:
- Every practice managing IDD should have a structured on-call system to facilitate communication between the managing clinician, implanting surgeon, and emergency department.
- Clinicians should educate patients on the signs of overdose, withdrawal, and other complications. In emergencies, direct communication with the managing physician is crucial to avoid redundant procedures.

More effective management of intrathecal drug delivery.
© Copyright 2025. All rights reserved.

More effective management of intrathecal drug delivery devices.
© Copyright 2025. All rights reserved.
