Factors Affecting
Intrathecal Medication Delivery
2024 Cancer PAIN
Factors Affecting
Intrathecal Medication Delivery
2024 CANCER PAIN
Contrary to traditional beliefs, CSF movement is largely limited to localized oscillations along the spine rather than active circulation168,169,170,171. This makes the spread of IT medications highly dependent on hydrodynamic and physicochemical factors, that can result in unpredictable drug distribution patterns172,173.
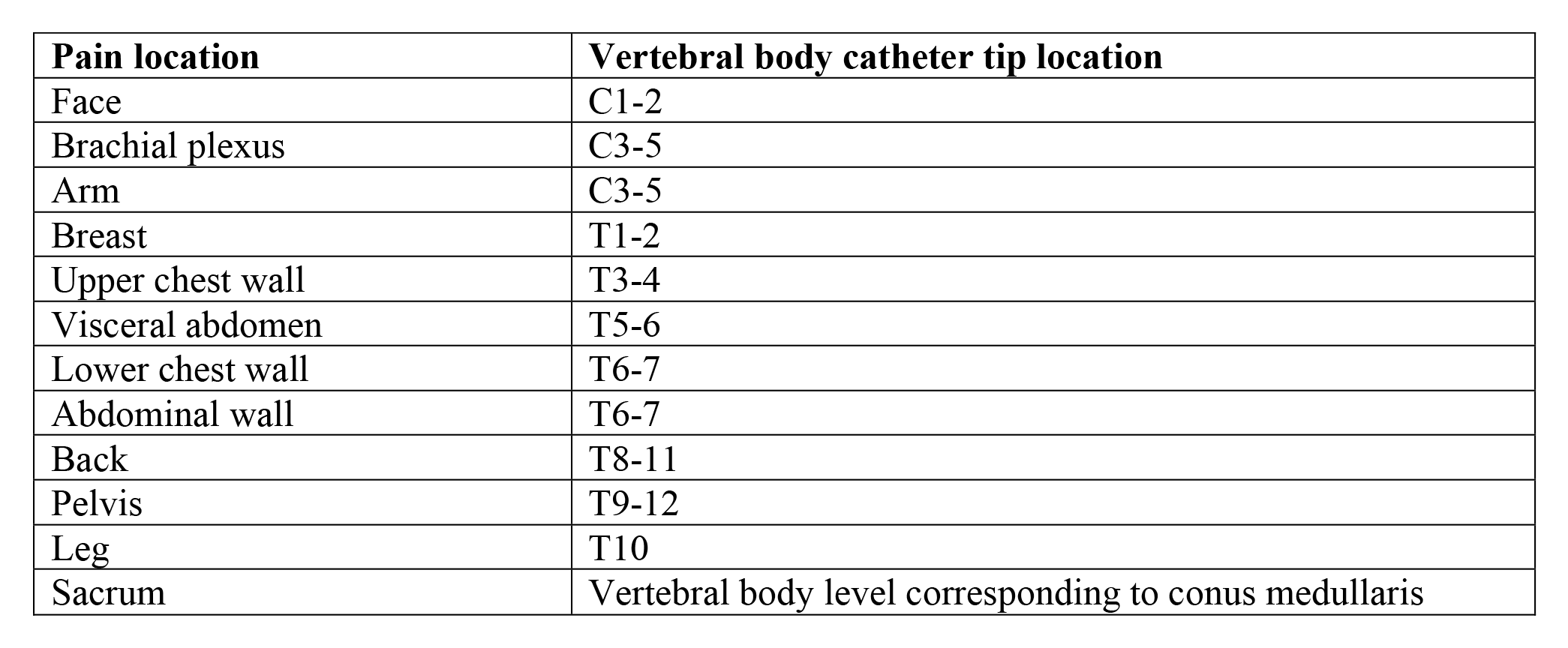
While previous guidelines recommended positioning the catheter tip at the spinal level corresponding to the painful area31,179,180, recent evidence suggests that exact placement (dorsal or ventral) may not significantly impact clinical outcomes181. More research is needed to determine optimal catheter positioning for different pain syndromes.
Table 7. Intrathecal Catheter Tip Location Based on Pain Location
Reprinted from Neuromodulation: Technology at the Neural Interface, Timothy R Deer, Salim M Hayek, Jay S Grider, Jason E Pope, Shane E Brogan, Amitabh Gulati, Jonathan M Hagedorn, Natalie Strand, Jennifer Hah, Tony L Yaksh, Peter S Staats, Christophe Perruchoud, Nebojsa Nick Knezevic, Mark S Wallace, Julie G Pilitsis, Tim J Lamer, Eric Buchser, Vishal Varshney, Jill Osborn, Vasudha Goel, Brian A Simpson, Jose A Lopez, Denis Dupoiron, Michael F Saulino, Gladstone C McDowell 2nd , Fabian Piedimonte, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain, Pages No., Copyright (2024), with permission with permission from the International Neuromodulation Society.
Dosing Considerations for Cancer Pain
The primary aim is rapid and effective pain relief, which contrasts with the approach in noncancer pain management that focuses on long-term sustainable analgesia. This means higher initial intrathecal (IT) doses and potentially more rapid dose escalation are warranted in cancer patients, prioritizing symptom control and quality of life in the final months or years.
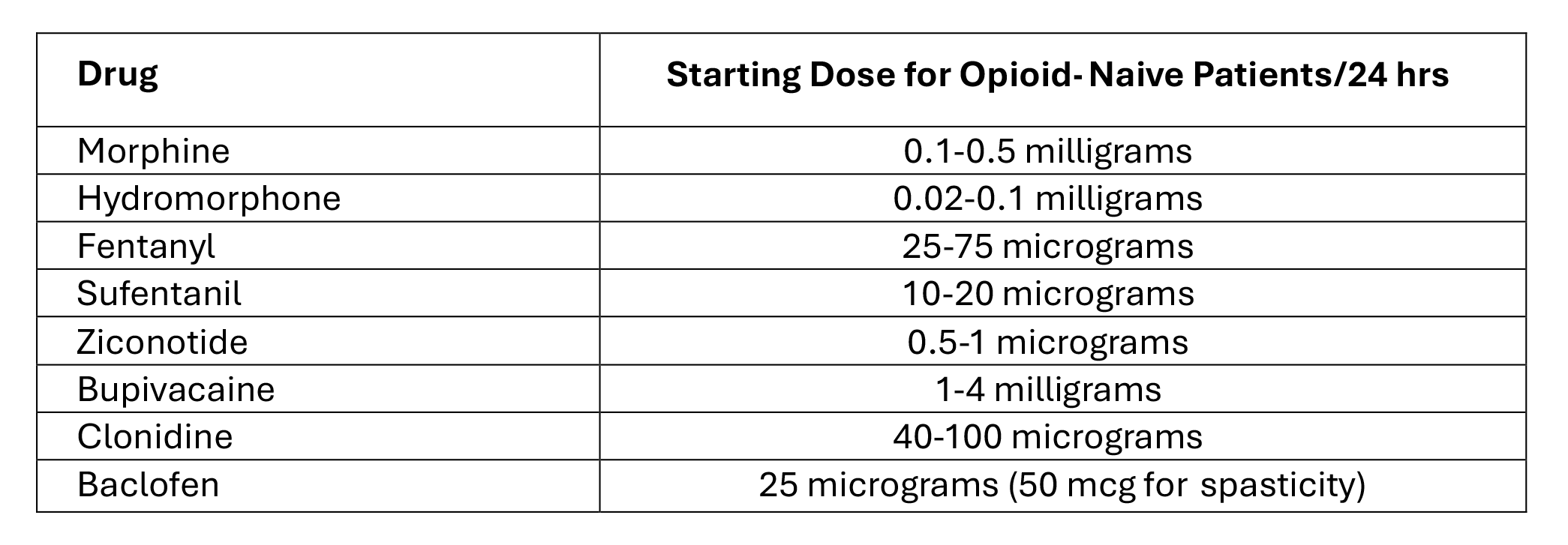
Initial IT opioid dosing in cancer pain can vary significantly based on the patient’s prior opioid usage, pain characteristics, and adjunct therapies. Concentrating the drug to maintain daily delivery between 0.1-0.5 mL is recommended, though clinical practices and patient needs can result in wider variability16.
A study by Sindt et al. proposed a systematic approach using a 100:1 ratio of oral morphine equivalent (OME) to IT opioid dose to ensure a smooth transition, assuming all systemic opioids are discontinued138. This ratio appears to be effective in avoiding underdosing, a common issue when transitioning from systemic to IT analgesia. Demand doses were set at 10% of the daily IT dose, which could be activated up to 24 times during hospitalization, and then decreased to a maximum of 12 activations per day upon discharge.
Dose adjustments the morning after implantation showed 42% of patients required no changes, 56% required dose increases, and only 4% needed a decrease due to non-serious side effects such as pruritus or nausea. No respiratory complications or withdrawal symptoms were reported, suggesting that an initial 100:1 ratio can effectively balance pain relief and safety in most patients.
The median OME in the study group was 240 mg, but for patients on higher systemic opioid doses, a more conservative transition strategy is advised to ensure patient safety and minimize the risk of overdose or adverse effects19.
Table 8. Starting and Dose Increases for Intrathecal Pain Medications in Patients Naïve to or on Low Doses of Opioids
Reprinted from Neuromodulation: Technology at the Neural Interface, Timothy R Deer, Salim M Hayek, Jay S Grider, Jason E Pope, Shane E Brogan, Amitabh Gulati, Jonathan M Hagedorn, Natalie Strand, Jennifer Hah, Tony L Yaksh, Peter S Staats, Christophe Perruchoud, Nebojsa Nick Knezevic, Mark S Wallace, Julie G Pilitsis, Tim J Lamer, Eric Buchser, Vishal Varshney, Jill Osborn, Vasudha Goel, Brian A Simpson, Jose A Lopez, Denis Dupoiron, Michael F Saulino, Gladstone C McDowell 2nd , Fabian Piedimonte, Robert M Levy, The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain, Pages No., Copyright (2024), with permission with permission from the International Neuromodulation Society.
Consensus Point 16. The PACC recommends that daily initiating IT dose be 50% or less of the successful bolus trial dose if an IT trial was performed, with demand dosages of 5% to 20% of the total daily dose. Other factors affecting initial IT doses include risks of cardiopulmonary depression and whether the patient will be admitted to the hospital for observation. However, these recommendations do not specifically consider the needs of the cancer pain population or patients on no or very low doses of opioids prior to trialing/implant. USPSTF Grade B; Level of certainty low; Quality of evidence II
Chronic use of IT opioids can lead to tolerance and complications such as hyperalgesia and lower extremity swelling186,187. Strategies like combining opioids with bupivacaine from the outset can help limit dose escalation and associated adverse effects.
Pump and Infusion Characteristics
While no significant updates have occurred since the 2017 guidelines, differences exist between peristaltic and valve-gated pump systems30,31. The choice of pump affects infusion accuracy and drug dispersion. Programmable pumps, though more expensive, offer flexibility to change regimens as the disease state evolves. With the potential need for MRIs in oncology patients clinical infrastructure and logistic plans need to be in place to deal with elective or urgent studies.
Drug Dispersion and Infusion Characteristics: The rostrocaudal spread of IT medications is influenced by factors like gravity, patient positioning, and cardiac cycle dynamics. Drug characteristics (e.g., hydrophilicity or lipophilicity) also play a role in distribution and clearance. For example, morphine (hydrophilic) has a longer residence time in CSF compared to fentanyl (lipophilic).
Consensus Point 17. The PACC recommends that understanding a drug’s pharmacokinetics in the IT space and the infusion characteristics of the drug delivery system will enhance the management and potential efficacy of IT drug delivery for pain control. USPSTF Grade B; Level of certainty low; Quality of evidence I-B
Deer TR, Hayek SM, Pope JE, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations for trialing of intrathecal drug delivery infusion therapy. Neuromodulation 2017;20(2):133-154.

More effective management of intrathecal drug delivery.
© Copyright 2025. All rights reserved.

More effective management of intrathecal drug delivery devices.
© Copyright 2025. All rights reserved.
